Are you looking for an answer to the topic “z in chemistry“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (np) or the number of protons found in the nucleus for every atom of that element.Why the atomic number is denoted by Z? The atomic number symbol, Z, stands for “Zahl,” meaning German number. The symbol Z denoted an element’s place in the periodic table before 1915.The atomic number, Z, is the number of protons in the nucleus. • This defines the chemical nature of the atom. • It is equal to the total charge on the nucleus.

Why atomic number is Z?
Why the atomic number is denoted by Z? The atomic number symbol, Z, stands for “Zahl,” meaning German number. The symbol Z denoted an element’s place in the periodic table before 1915.
What is Z in chemistry energy?
The atomic number, Z, is the number of protons in the nucleus. • This defines the chemical nature of the atom. • It is equal to the total charge on the nucleus.
cis-trans and E-Z naming scheme for alkenes | Alkenes and Alkynes | Organic chemistry | Khan Academy
Images related to the topiccis-trans and E-Z naming scheme for alkenes | Alkenes and Alkynes | Organic chemistry | Khan Academy

What is Z for an electron?
Z = denotes the number of protons existing in the nucleus. S = average amount of density between the nucleus and the electron.
What is Z and a in chemistry?
Z = atomic number = number of protons in the nucleus = number of electrons orbiting the nucleus; A = mass number = number of protons and neutrons in the most common (or most stable) nucleus.
Where is Z on the periodic table?
Atomic Number (Z)
It is not the atom’s relative mass, as we will see in the section on isotopes below. It is, rather, the number of protons in the nucleus, which we call the atomic number and denote by the symbol Z.
What is Z in quantum chemistry?
Z is called the atomic number. The more Z is high, the more complex to study the atom becomes. The electrons interact both with each other and with protons of nucleus.
What is Z in Bohr’s equation?
B = Bohr’s energy (2.178×10-18 J) Z = charge of the nucleus (H = +1, Li = +3) n = main quantum number; associated to the level of the electron orbit. small value of n = electron closer to the nucleus lower energy level.
See some more details on the topic z in chemistry here:
What does Z in chemistry mean? – Quora
In chemistry and physics, the atomic number of a chemical element (also known as its proton number) is the number of protons found in the nucleus of an atom …
Atomic Mass and Mass Number Chemistry Review – ThoughtCo
Z is used to signify the atomic number or proton number of an atom · Z = # of protons of an atom · A is used to signify the atomic mass number ( …
Question: What Is Z In Chemistry – Seniorcareto
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
Why is atomic number represented by Z? | Socratic
The symbol for atomic number, Z, stands for “Zahl”, which means number in German. Prior to 1915, the symbol Z denoted the position of an …
What does Ze mean in physics?
Atoms can be arranged in the Periodic Table. Atomic nuclei have a charge +Ze, where e is the magnitude of the charge on an electron and Z is the atomic number. Z determines the number of atomic electrons and hence the ordered position of that species of atom in the periodic table.
What is the Z of water?
Zn is the atomic number of each element. An example is that of water (H2O), made up of two hydrogen atoms (Z=1) and one oxygen atom (Z=8), the total number of electrons is 1+1+8 = 10, so the fraction of electrons for the two hydrogens is (2/10) and for the one oxygen is (8/10).
What is Z effective of sodium?
Now, for sodium : 1s², 2s², 2p⁶, 3s¹ σ =0.35 ×0 + 0.85 × 8 + 1 × 2 = 0 + 6.8 + 2 = 8.8. So, Zeff = 11 – 8.8 = 2.2.
How To Calculate The Effective Nuclear Charge of an Electron
Images related to the topicHow To Calculate The Effective Nuclear Charge of an Electron
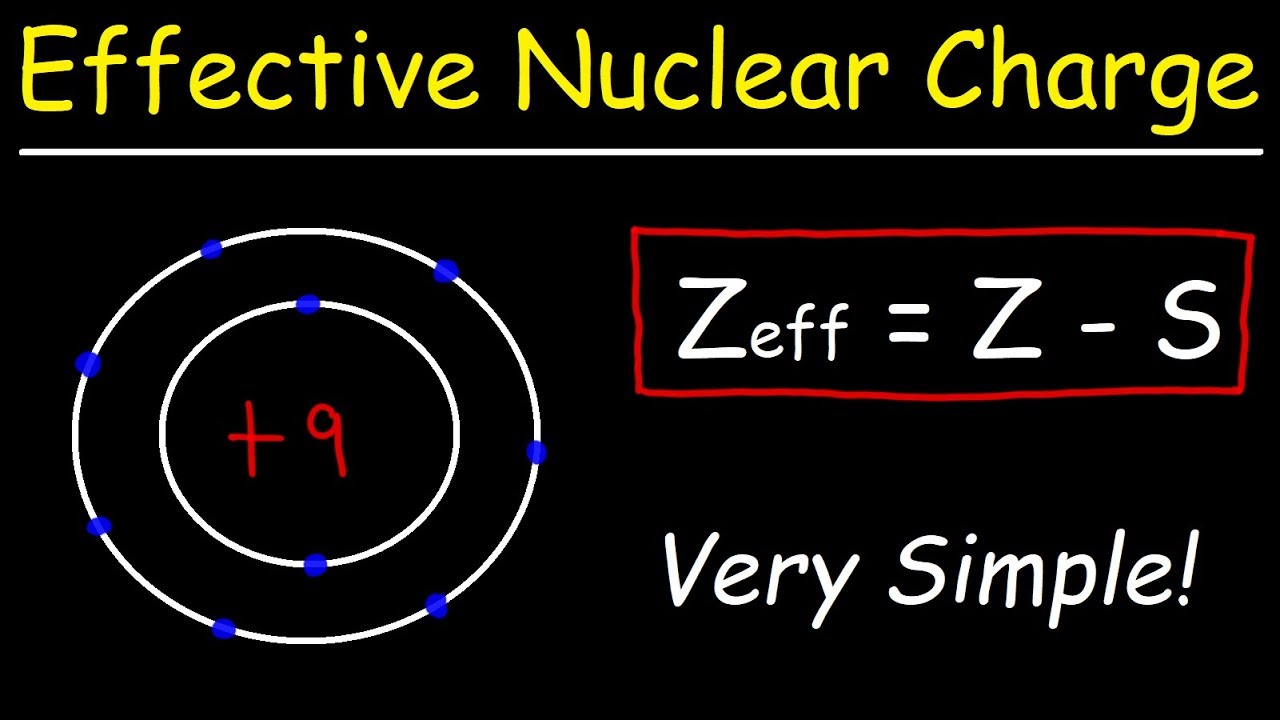
What is Zeff trend?
Going across a period, Effective Nuclear Charge (Zeff) increases. Distance and shielding remain constant. – causing those atoms to be more compact. Electronegativity Electronegativity is the ability of an atom to attract electrons while forming a bond in a compound.
What does Z stand for in organic chemistry?
Z: Describes the configuration of a double bond in which the two groups of highest Cahn-Ingold-Prelog priority are cis. From the German zusammen, meaning together.
What is Z in chemistry class 11?
Hint:According to Coulomb’s law, the attraction of an electron to the nucleus depends only on the charge of an electron (+Z), the charge of the electron (-1) and the distance between them. The amount of positive charge of the nucleus experienced by any individual electron is an effective nuclear charge.
What is Z in Iupac name?
If both substituents ranked 1 are on the same side of the pi bond, the bond is given the descriptor Z (short for German Zusammen, which means “together”).
Is Z the atomic number?
The atomic number (represented by the letter Z) of an element is the number of protons in the nucleus of each atom of that element.
Which number is expressed by Z?
This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element. The symbol for the atomic number is designated with the letter Z. For example, the atomic number (z) for sodium (Na) is 11. That means that all sodium atoms have 11 protons.
What is the mass number of ZR?
Atomic mass of Zirconium is 91.224 u.
Note that each element may contain more isotopes. Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance.
How do you find the Z component of angular momentum?
The direction of angular momentum is quantized, in that its component along an axis defined by a magnetic field, called the z-axis is given by Lz=mlh2π(ml=−l,−l+1,…,−1,0,1,…l−1,l) L z = m l h 2 π ( m l = − l , − l + 1 , … , − 1 , 0 , 1 , … l − 1 , l ) , where Lz is the z-component of the angular momentum and ml is the …
Computational Chemistry 1.10 – Z-Matrix Format
Images related to the topicComputational Chemistry 1.10 – Z-Matrix Format

What is 1s 2s and 2p for orbitals?
At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. A p orbital is rather like 2 identical balloons tied together at the nucleus.
What is wavefunction in chemistry?
A wave function (Ψ) is a mathematical function that relates the location of an electron at a given point in space (identified by x, y, and z coordinates) to the amplitude of its wave, which corresponds to its energy.
Related searches to z in chemistry
- atomic mass
- zeeman effect in chemistry
- periodic table
- z in chemistry stands for
- z effect in chemistry
- zero point energy in chemistry
- zinc in chemistry
- zeolite in chemistry
- zeroth law of thermodynamics in chemistry
- meaning of z in chemistry
- isotopes
- ze in chemistry
- Atomic mass
- zeff in chemistry
- Periodic table
- what is m/z in chemistry
- atomic number la gi
- atomic number
- what is z in chemistry
- neutrons have
- e and z in chemistry
- group z in chemistry
- symbol z in chemistry
- value of z in chemistry
- Isotopes
- what is z in chemistry solid state
- Atomic number
- z represents in chemistry
- z means in chemistry
- n and z in chemistry
- how to find z in chemistry
Information related to the topic z in chemistry
Here are the search results of the thread z in chemistry from Bing. You can read more if you want.
You have just come across an article on the topic z in chemistry. If you found this article useful, please share it. Thank you very much.
