Are you looking for an answer to the topic “why do balmer lines of hydrogen get closer“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
This is because the energy levels (the “energy eigenvalues”) of the hydrogen atom get closer together as the energy increases.The intensity of spectral lines decreases as you move up the energy scale (or frequency scale) because there are fewer and fewer excited hydrogen atoms at high energies that decay directly to the ground state.The lines grow closer and closer together as the frequency increases. Eventually, they are so close together that it becomes impossible to see them as anything other than a continuous spectrum. This is suggested by the shaded part on the right end of the series.

Why do the Balmer lines become weaker toward shorter wavelengths?
The intensity of spectral lines decreases as you move up the energy scale (or frequency scale) because there are fewer and fewer excited hydrogen atoms at high energies that decay directly to the ground state.
Why do spectral lines get closer together?
The lines grow closer and closer together as the frequency increases. Eventually, they are so close together that it becomes impossible to see them as anything other than a continuous spectrum. This is suggested by the shaded part on the right end of the series.
The Balmer Series | Spectral Emission Lines of Diffuse Hydrogen Gas | Doc Physics
Images related to the topicThe Balmer Series | Spectral Emission Lines of Diffuse Hydrogen Gas | Doc Physics

Why do spectral lines in hydrogen atom become closer together farther away from the nucleus?
stars and stellar spectra
Spectral lines are produced by transitions of electrons within atoms or ions. As the electrons move closer to or farther from the nucleus of an atom (or of an ion), energy in the form of light (or other radiation) is emitted or absorbed.…
What did the Balmer series suggest about hydrogen?
Niels Bohr proposed a model for the hydrogen atom in 1913 that described discrete energy states are associated with a fixed electron orbit around the nucleus. Importantly, an atom cannot discharge energy while its electrons are in stationary states.
What is the shortest wavelength of Balmer series of hydrogen?
The shortest wavelength in the Balmer series is (R = 1.097 × 107 m-1)
What is the shortest wavelength of Balmer series in hydrogen spectrum?
In hydrogen spectrum, the shortest wavelength in Balmer series is the ‘lambda ‘ .
Why do energy levels get closer?
Unlike a ladder, which has a limited length, the energy levels of an atom extend infinitely out from the nucleus and the energy levels are not evenly spaced. As the distance from the nucleus increases, the levels get closer together and contain more-energetic electrons (Figure 5.4).
See some more details on the topic why do balmer lines of hydrogen get closer here:
Balmer series – Wikipedia
The Balmer series, or Balmer lines in atomic physics, is one of a set of six named series describing the spectral line emissions of the hydrogen atom.
Why the distance between the emission lines of hydrogen …
The lines grow closer and closer together as …
Why Do The Balmer Lines Of Hydrogen Get Closer Together …
why do the balmer lines of hydrogen get closer together as you go towards shorter wavelengths? · Emission spectrum of hydrogen | Chemistry | Khan …
Balmer Series Definition in Science – ThoughtCo
The hydrogen emission spectrum is the Balmer series. … There are also multiple ultraviolet Balmer lines that have wavelengths shorter than …
What do you mean by Stark effect?
Stark effect, , the splitting of spectral lines observed when the radiating atoms, ions, or molecules are subjected to a strong electric field. The electric analogue of the Zeeman effect (i.e., the magnetic splitting of spectral lines), it was discovered by a German physicist, Johannes Stark (1913).
When the wavelength of spectral lines emitted from an object increases which end of the visible light spectrum does it move toward?
When the wave length of spectral lines emitted from an object increases, which end of the visible light spectrum does it move toward, and what is the object’s motion relative to Earth? The light spectrum it moves toward when an object increases is the red shift, and it is moving away from earth.
Why do lines converge at higher frequency?
If this photon falls into the visible spectrum of light, then it produces a visible spectrum. As electrons move further away from the nucleus, the electron shells become closer together in terms of space and energy, and so lines converge towards the end of the spectrum.
How does Bohr model explain the line spectrum of hydrogen?
Bohr’s model explains the spectral lines of the hydrogen atomic emission spectrum. While the electron of the atom remains in the ground state, its energy is unchanged. When the atom absorbs one or more quanta of energy, the electron moves from the ground state orbit to an excited state orbit that is further away.
How does the emission spectrum of hydrogen compare to its absorption spectrum?
Emission of Light by Hydrogen
This is why hydrogen’s emission spectrum is the inverse of its absorption spectrum, with emission lines at 410 nm (violet), 434 nm (blue), 486 nm (blue-green), and 656 nm (red). The highest energy and shortest wavelength light is given off by the electrons that fall the farthest.
Emission spectrum of hydrogen | Chemistry | Khan Academy
Images related to the topicEmission spectrum of hydrogen | Chemistry | Khan Academy

What is Balmer formula How does it explain the line spectrum of hydrogen?
Johann Balmer, a Swiss mathematician, discovered (1885) that the wavelengths of the visible hydrogen lines can be expressed by a simple formula: the reciprocal wavelength (1/λ) is equal to a constant (R) times the difference between two terms, 1/4… In principles of physical science: Compilation of data.
What electron transitions make up the Balmer series?
Balmer series, or Balmer lines, are the visible part of the spectrum corresponding to the electron transitions in Hydrogen atom. More precisely, Balmer series correspond to jumps from d, e, f, … energy levels (n≥ 3, with n being the principal quantum number) onto the p energy levels (n=2).
Why are there so few lines in hydrogen compared to other atoms?
Though a hydrogen atom has only one electron, it contains a large number of shells, so when this single electron jumps from one shell to another, a photon is emitted, and the energy difference of the shells causes different wavelengths to be released… hence, mono-electronic hydrogen has many spectral lines.
What is the longest and shortest wavelength in Balmer series?
- A. 911.7 oA and 1215.7 oA.
- B. 3647 oA and 6565 oA.
- C. 6565oA and 3647 oA.
- D. 911.7 oA and 6565 oA.
What is the longest wavelength in the Balmer series?
Thus the longest wavelength in the Balmer series is 656 nm.
What is the ratio of the shortest wavelength of Balmer series to the shortest wavelength of Lyman series?
What is the ratio of shortest wavelength of the Balmer series ot the shortest waelength of the Lyman series? The ratio of shortest wavelength lines in Lyman , Balmer and Paschen series is 1:4:x.
Does the whole Balmer series fall in the visible region?
The spectral lines of Balmer series fall in the visible region for the Hydrogen atom.
What is the wavelength range of Balmer series of hydrogen atom?
…
Balmer series (n′ = 2)
| n | λ, air (nm) |
|---|---|
| 7 | 397.0 |
| ∞ | 364.6 |
| Source: |
Which has the longest wavelength?
Radio waves have the longest wavelength, and gamma rays have the shortest wavelength. Encyclopædia Britannica, Inc.
Why are orbitals closer to the nucleus lower in energy?
In the lowest energy level, only one orbital exists which can carry a maximum of two electrons. Because more protons are present in the nucleus, the force of attraction between the protons and electrons is stronger. This results in the electrons closest to the nucleus having less energy.
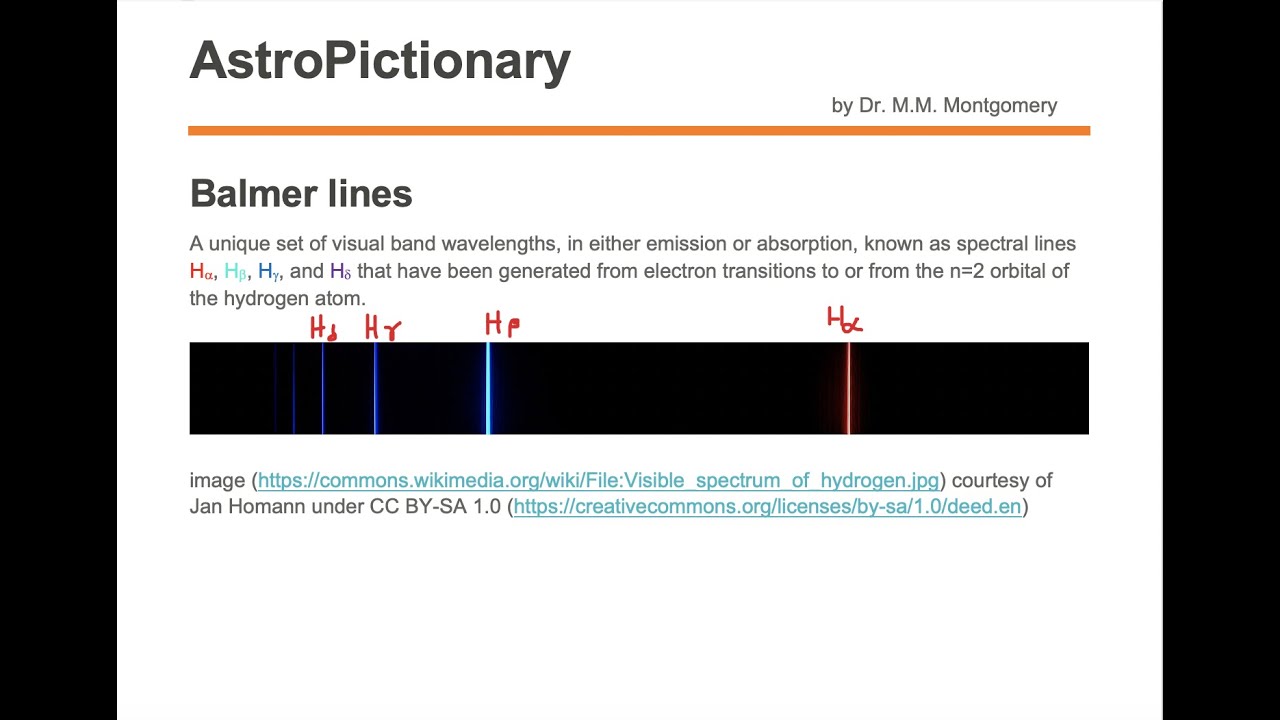
Balmer lines
Images related to the topicBalmer lines
Do orbitals closer to the nucleus have more energy?
Energy levels consist of orbitals and sub-orbitals. The lower the energy level the electron is located at, the closer it is to nucleus.
What happens when electrons move closer to the nucleus?
Answer and Explanation: When the electron moves closer to the nucleus, the magnitude of energy of the nucleus increases.
Related searches to why do balmer lines of hydrogen get closer
- emission spectrum
- Emission spectrum
- Rydberg formula
- Balmer series
- Hydrogen line
- H alpha
- be star
- spectral line
- balmer series
- history of hydrogen
- h alpha
- rydberg formula
- hydrogen line
- History of hydrogen
Information related to the topic why do balmer lines of hydrogen get closer
Here are the search results of the thread why do balmer lines of hydrogen get closer from Bing. You can read more if you want.
You have just come across an article on the topic why do balmer lines of hydrogen get closer. If you found this article useful, please share it. Thank you very much.
