Are you looking for an answer to the topic “why are spectral lines often referred to as atomic fingerprints“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Why are spectral lines often referred to as “atomic fingerprints”? Because if you see a specific line in a spectrum, you know that exact element that produces that line is present.A spectral line is like a fingerprint that can be used to identify the atoms, elements or molecules present in a star, galaxy or cloud of interstellar gas. If we separate the incoming light from a celestial source using a prism, we will often see a spectrum of colours crossed with discrete lines.“The spectral lines for atoms are like fingerprints for humans.” How do the spectral lines for Hydrogen and Boron support this statement? Electrons and protons (attract/repel) each other. As an electron gets closer to the nucleus the (attraction/repulsion) to the nucleus gets (stronger/weaker).

Why are spectral lines called atomic fingerprints?
A spectral line is like a fingerprint that can be used to identify the atoms, elements or molecules present in a star, galaxy or cloud of interstellar gas. If we separate the incoming light from a celestial source using a prism, we will often see a spectrum of colours crossed with discrete lines.
How are spectral lines for atoms like fingerprints?
“The spectral lines for atoms are like fingerprints for humans.” How do the spectral lines for Hydrogen and Boron support this statement? Electrons and protons (attract/repel) each other. As an electron gets closer to the nucleus the (attraction/repulsion) to the nucleus gets (stronger/weaker).
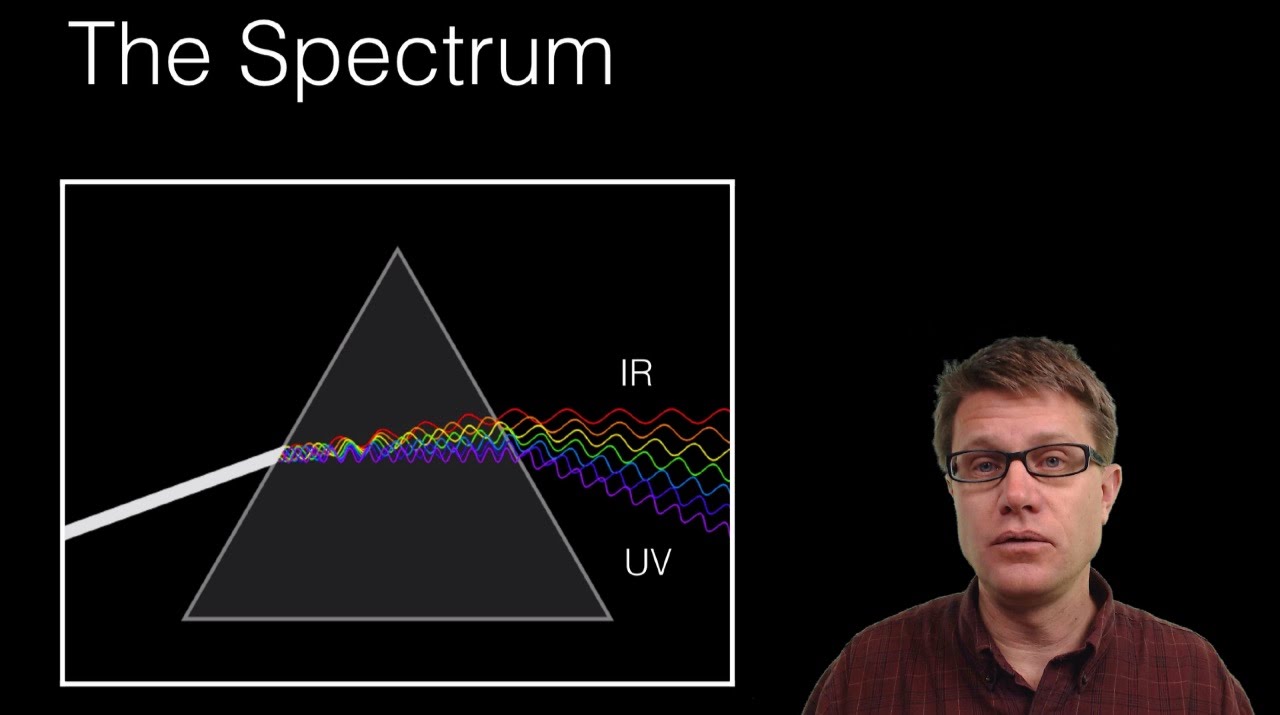
Spectral Lines of Hydrogen Atom
Images related to the topicSpectral Lines of Hydrogen Atom

What is an atomic fingerprint?
“atomic fingerprint” A term for the unique line spectrum that is characteristic of a given element and can be used for identification. Balmer formula. A mathematical equation for calculating the emitted wavelength of a light from an excited hydrogen atom when an electron drops to the second energy level.
Why are line emission spectra elements called atomic fingerprints Quizizz?
Why are line emission spectra of elements called “atomic fingerprints”? All stars are composed of a mixture of elements. When these elements are heated they emit specific amounts of electromagnetic radiation, known as an emission spectrum. Each element emits a unique, identifiable, spectrum.
Which spectra is known as fingerprint spectra?
IR spectra is called the fingerprint region, because the absorption pattern is highly complex but unique to each organic structure. The stretching vibrations for both the carbon-carbon and carbon-oxygen double bonds are easily identified at 6.1 and 5.8 μm, respectively.
What produces the atomic fingerprint of an element?
Electrons exist in energy states within the atom (called orbitals by chemists). When an electron jumps between energy levels of an atom it either absorbs energy for the transition or emits a photon when reducing its potential energy.
Emission and Absorption Spectra
Images related to the topicEmission and Absorption Spectra
See some more details on the topic why are spectral lines often referred to as atomic fingerprints here:
Why are spectral lines often referred to as atomic fingerprints?
There is no same energy level difference from one atom to another, therefore spectral lines are referred to as an “atom’s fingerprint”.
Spectral Line | COSMOS – Centre for Astrophysics and …
A spectral line is like a fingerprint that can be used to identify the atoms, … Note that spectral lines can also occur in other regions of the …
Why is line spectra also regarded as the ‘finger prints of atoms’
Each element has a unique line emission spectrum. The characteristic lines in atomic spectra can be used in chemical analysis to identify unknown atoms in …
Why is a line spectrum called the atomic fingerprint? – Sulfonte
? Spectral lines are often referred to as the stars’ “fingerprints” because: …
What do spectral lines tell us?
From spectral lines astronomers can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star. The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this.
What acts like fingerprints that identify atoms?
Physicists in Japan, Spain and the Czech Republic have developed a new type of atomic force microscope (AFM) that can “fingerprint” the chemical identity of individual atoms on a material’s surface. This is one step ahead of existing AFMs, which can only detect the position of atoms.
How a spectral fingerprint for an element is formed?
If the light emitted from the excited atoms is viewed through a prism, then individual patterns of lines will be produced. These lines are called spectra and correspond to fingerprint wavelengths (symbol for wavelength is λ) for a specific element.
Which of the following spectra is produced by the sun?
The full electromagnetic spectrum. The spectrum of the Sun appears as a continuous spectrum and is frequently represented as shown below. This type of spectrum is called an emission spectrum because what you are seeing is the direct radiation emitted by the source.
Spectral line
Images related to the topicSpectral line

What did Bohr’s model explain?
Summary. The Bohr model postulates that electrons orbit the nucleus at fixed energy levels. Orbits further from the nucleus exist at higher energy levels. When electrons return to a lower energy level, they emit energy in the form of light.
What is the first man made element discovered in 1973?
In 1973, mendelevium(I) was reported to have been produced by Russian scientists, who obtained it by reducing higher oxidation states of mendelevium with samarium(II).
Related searches to why are spectral lines often referred to as atomic fingerprints
- the broadening of spectral lines can be caused by
- why are spectral lines from the bright line spectrum referred to as “fingerprints” of the atoms?
- what information about an astronomical object can be determined by observing its spectrum
- why are spectral lines like fingerprints
- the doppler effect causes spectral lines to
- the spectral lines for atoms are like fingerprints
- what information about an astronomical object can be determined by observing its spectrum?
- why are spectral lines from the bright line spectrum referred to as fingerprints of the atoms
- why the atomic number is called the fingerprint of elements
- which of the following can produce a continuous spectrum
Information related to the topic why are spectral lines often referred to as atomic fingerprints
Here are the search results of the thread why are spectral lines often referred to as atomic fingerprints from Bing. You can read more if you want.
You have just come across an article on the topic why are spectral lines often referred to as atomic fingerprints. If you found this article useful, please share it. Thank you very much.