Are you looking for an answer to the topic “which type of bond is found in sodium bromide“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and that the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom.Bromine atoms contain 35 electrons. Bromine exists as a diatomic molecule (Br 2) and has 70 electrons in total. Bromine molecules contain pure covalent bonds so are held together by London dispersion forces (temporary dipoles caused by momentary uneven sharing of electrons.
What bond is present in bromine and bromine?
Bromine atoms contain 35 electrons. Bromine exists as a diatomic molecule (Br 2) and has 70 electrons in total. Bromine molecules contain pure covalent bonds so are held together by London dispersion forces (temporary dipoles caused by momentary uneven sharing of electrons.
Is NaBr ionic or covalent bond?
Is sodium bromide a covalent or ionic? Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and that the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom.
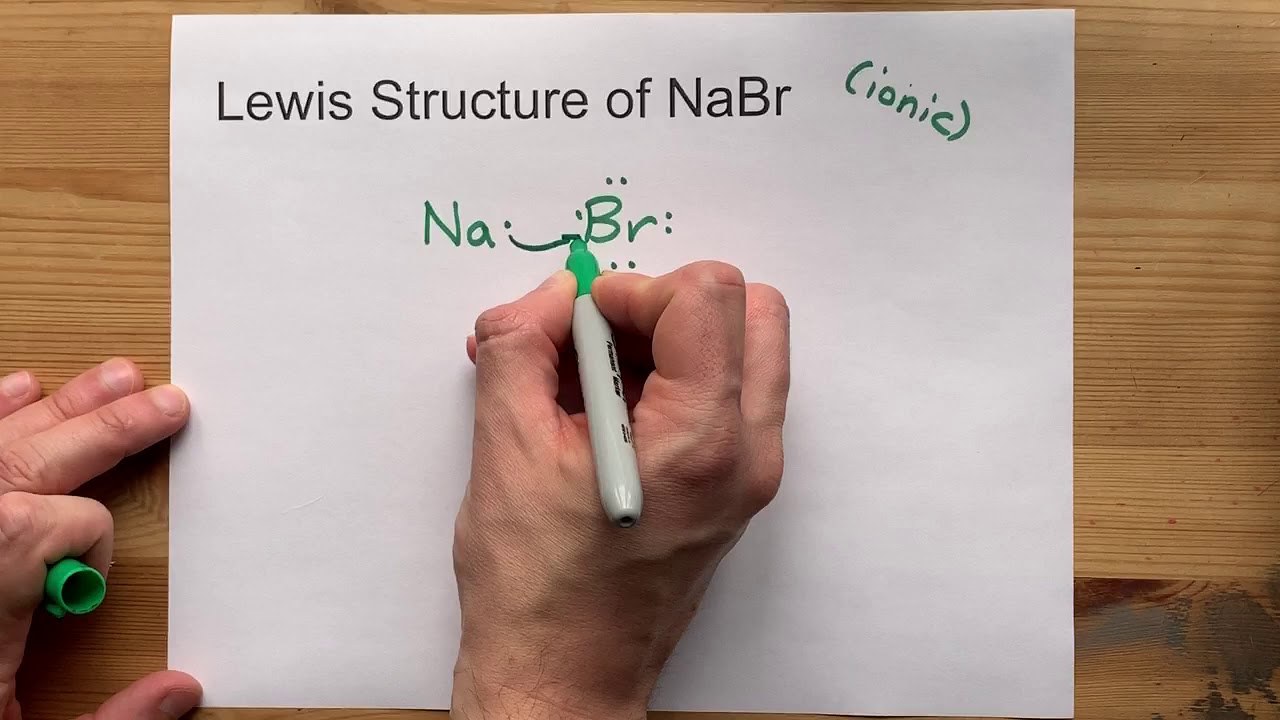
Draw the Lewis Structure of NaBr (sodium bromide)
Images related to the topicDraw the Lewis Structure of NaBr (sodium bromide)

What is the polarity compound of sodium and bromine?
Ionically bonded sodium bromide is a chemical compound. Bromine has a high electronegativity, which means that the electromagnetic force between the Br and Na atoms is strong enough to transfer one electron from the Na to the Br atom. Bromine is negatively charged as a result, whereas sodium is positively charged.
What is a polar covalent bond?
Polar Covalent Bonds. A polar covalent bond exists when atoms with different electronegativities share electrons in a covalent bond. Consider the hydrogen chloride (HCl) molecule. Each atom in HCl requires one more electron to form an inert gas electron configuration.
What are the two types of covalent bonds?
There are two basic types of covalent bonds: polar and nonpolar. In a polar covalent bond, the electrons are unequally shared by the atoms and spend more time close to one atom than the other.
How is NaBr ionic bond formed?
Formula and structure: Sodium bromide chemical formula is NaBr and the molar mass is 102.89 g mol–1. It can be found hydrated by one (monohydrate NaBr) or two (duhydrate NaBr) water molecules. Sodium bromide is formed by one sodium cation Na+ and one bromide anion Br– which are joined through an ionic bond.
Is NaCl ionic or covalent?
Ionic bonds usually occur between metal and nonmetal ions. For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. In a covalent bond, the atoms bond by sharing electrons. Covalent bonds usually occur between nonmetals.
See some more details on the topic which type of bond is found in sodium bromide here:
What bond attaches to sodium bromide? – Quora
NaBr is an ionic compound. · Bromine has enough electronegativity that the electromagnetic force between the Br and the Na atoms is great enough that an electron …
Which type of bond is found in sodium bromide? – Sawaal
Which type of bond is found in sodium bromide? A) Ionic, B) Covalent. C) Polar covalent, D) None of the above. Answer: A) Ionic Explanation:.
Sodium bromide | NaBr – PubChem
Sodium bromide | NaBr or BrNa | CID 253881 – structure, chemical names, … on List D. Sodium bromide is found on List D. Case No: 4051; Pesticide type: …
Types of bonds – crystal – Encyclopedia Britannica
The sodium atom has a single electron in its outermost shell, … A covalent bond is formed by two electrons—one from each atom—located in orbitals between …
Which compound contains both ionic and covalent bonds?
Calcium carbonate is another example of a compound with both ionic and covalent bonds. Here calcium acts as the cation, with the carbonate species as the anion. These species share an ionic bond, while the carbon and oxygen atoms in carbonate are covalently bonded.
Which compound contains covalent bonds?
Examples of compounds that contain only covalent bonds are methane (CH4), carbon monoxide (CO), and iodine monobromide (IBr). Covalent bonding between hydrogen atoms: Since each hydrogen atom has one electron, they are able to fill their outermost shells by sharing a pair of electrons through a covalent bond.
Is NaBr Ionic or Covalent? (Sodium bromide)
Images related to the topicIs NaBr Ionic or Covalent? (Sodium bromide)
Which compound contains ionic bonds?
Ionic compounds contain ions and are held together by the attractive forces among the oppositely charged ions. Common salt (sodium chloride) is one of the best-known ionic compounds. Molecular compounds contain discrete molecules, which are held together by sharing electrons (covalent bonding).
Is HBr polar covalent or nonpolar covalent?
Is HBr a polar or nonpolar molecule? Because of the electronegativity value difference between hydrogen atom (2.2) and bromine atom (2.96), hydrogen bromide (HBr) is a polar molecule (dipole moment 2.60 D).
Is bromine ionic?
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table.
Is bromine and hydrogen covalent bond?
Both the hydrogen and the bromine can count the two electrons in the bond as its own because the electrons are shared between both atoms. Hydrogen needs only two electrons to fill its valence, which it gets through the covalent bond.
Is sodium sulfide ionic or covalent?
Sodium sulfide compound is an ionic compound. There are 2 Na atoms per 1 Sulfide atom. It has a central Sulfur atom encircled by 4 Oxygens in covalent bonds. The Sodium atoms and Sulfur or Oxygen atoms in the compound exchange their electrons.
Is NaF polar or nonpolar?
One sodium-fluorine single bonds in the sodium fluoride(NaF), for example, are polarised toward the more electronegative value fluorine atom, and because (Na-F) single bonds have the same size and polarity, their sum is nonzero due to the NaF molecule’s bond dipole moment due to pulling the electron cloud to the two …
Is NaCl a nonpolar molecule?
Note ionic compounds, such as sodium chloride (NaCl), are polar. However, most of the time when people talk about “polar molecules” they mean “polar covalent molecules” and not all types of compounds with polarity!
How to Write the Formula for NaBr (Sodium bromide)
Images related to the topicHow to Write the Formula for NaBr (Sodium bromide)

Is CH4 a polar covalent bond?
2 Answers By Expert Tutors. Methane contains nonpolar covalent bonds. This is because there is a very small difference in the electronegativity of Carbon and Hydrogen. Thus they are going to share their electrons fairly equally.
Is HBr polar or nonpolar?
Is HBr a polar or nonpolar molecule? Because of the electronegativity value difference between hydrogen atom (2.2) and bromine atom (2.96), hydrogen bromide (HBr) is a polar molecule (dipole moment 2.60 D).
Related searches to which type of bond is found in sodium bromide
- what type of bond is present in csbr?
- what type of bonding is in sodium
- what type of bond is k-oh
- what type of bond is sodium
- sodium and aluminum bond type
- magnesium bromide type of bond
- what type of bond is hcl
- what type of bond is co
- what type of chemical bonds does sodium bromide have ionic or covalent
- what type of bond is present in inorganic salt
- what type of bond is k oh
- what type of bond is nf
- what type of bond is present in csbr
- what type of bond is found in sodium chloride
- what type of bond is licl
Information related to the topic which type of bond is found in sodium bromide
Here are the search results of the thread which type of bond is found in sodium bromide from Bing. You can read more if you want.
You have just come across an article on the topic which type of bond is found in sodium bromide. If you found this article useful, please share it. Thank you very much.