Are you looking for an answer to the topic “which of the following liquids will have the highest freezing point?“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Keep Reading

What is the highest freezing point of a liquid?
The highest freezing point will be the one with the lowest molality of particles of solute, the glucose solution; the next highest will be the Ca(NO3)2 solution; and the lowest freezing point will be the KCl solution.
Which of the following will have highest freezing point?
As \[FeC{l_3}\] solution comprises the maximum number of solute particles, its freezing point will be the lowest and sugar solution comprises the minimum number of solute particles so its freezing point will be the highest. So, the correct answer is Option B.
Which of the following solution will have highest freezing point?
Images related to the topicWhich of the following solution will have highest freezing point?

Which solution has the highest freezing point quizlet?
CH3CH2OH is non-electrolyte and doesn’t dissociate into ions and therefore it’s 1.5m concentration is the lowest concentration of dissolved species amongst the answer choices and this solution will have the smallest decrease (depression) in its freezing point leaving it with the overall highest freezing point.
Which of the following solution will have highest freezing point depression?
Al2(SO4)3 solution contains more number of particles than NaCl solution. Hence, Al2(SO4)3 solution has maximum ΔTf. Therefore, the freezing point depression of 0.05 m Al2(SO4)3 solution will be higher than 0.1 m NaCl solution.
Which solution has maximum freezing point in a same situation?
Solution : 0.01 M urea will have maximum freezing point because it will have the lowest depression in freezing point ( being non-electrolyte).
Which of the following has highest freezing point 0.1 M urea?
Hence, the urea solution will have highest freezing point.
How do you find the highest and lowest freezing point?
Multiply the original molality (m) of the solution by the number of particles formed when the solution dissolves. This will give you the total concentration of particles dissolved. Compare these values. The higher total concentration will result in a higher boiling point and a lower freezing point.
See some more details on the topic which of the following liquids will have the highest freezing point? here:
Solved which of the following liquids will have the highest
which of the following liquids will have the highest freezing point? A) pure H2O B) aqueous Fel3 (0.24 m) C) aqueous NaCl (0.50 m)
Which of the following liquids will have the highest freezing …
Answer to: Which of the following liquids will have the highest freezing point? a. aqueous FeI3 (0.030 m) b. aqueous CoI2 (0.030 m) c. aqueous NaI (0.030 m) …
Which of the following liquids will have the lowest freezing …
Which of the following liquids will have the lowest freezing point? aqueous LiF (0.65 m) pure H2O … aqueous sucrose ( 0.75 m ) aqueous CdI2(0.39m) aqueous …
Which of the following liquids will have the lowest … – Socratic
According to colligative properties of solutes, depression of freezing point follows the number of particles produced upon solubilization.
What is freezing point of water?
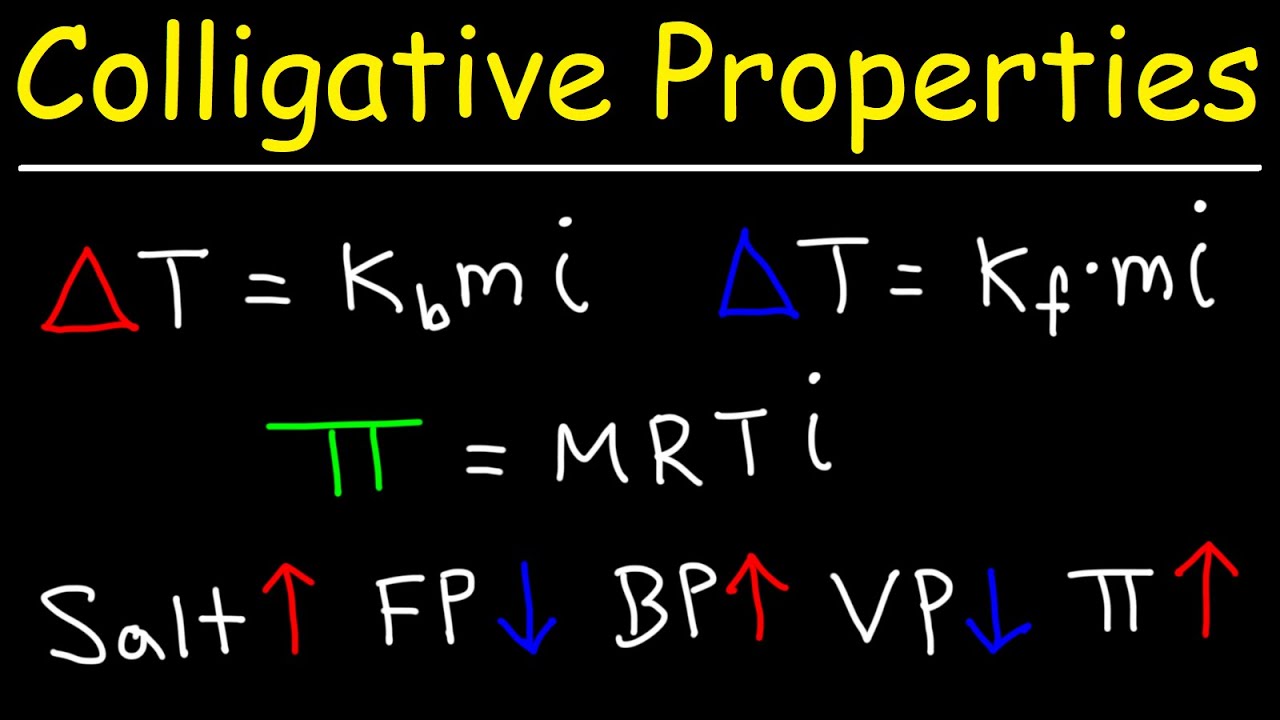
Colligative Properties – Boiling Point Elevation, Freezing Point Depression Osmotic Pressure
Images related to the topicColligative Properties – Boiling Point Elevation, Freezing Point Depression Osmotic Pressure
What is freezing point?
Medical Definition of freezing point
: the temperature at which a liquid solidifies specifically : the temperature at which the liquid and solid states of the substance are in equilibrium at atmospheric pressure : melting point the freezing point of water is 0° Celsius or 32° Fahrenheit.
Which of the following has the lowest freezing point h2o N2 so2 H2 co2?
H2 has the lowest freezing point. Which has the smallest vapor pressure at 25oC?
Which solution will freeze at the lowest temperature?
The addition of excess of solute to the solution decreases the freezing point. This means that the solution with a higher concentration will have the lowest freezing point.
Which of the following has the lowest freezing point?
The correct option is c. Methane has the lowest freezing point in all the above-given options.
Which of the following will have highest freezing point depression and why?
Depression in freezing point is a colligative property which depends on number of particles. Among given choices K2SO4 gives maximum number of ions, so it will have maximum depression in freezing point.
Which of the following has higher freezing point 0.05 m Al2 SO4 3?
The correct answer is – 0.05 M Al₂(SO₄)₃ has a higher freezing point. Al₂(SO₄)₃ dissociates in water to give 5 mol of ions per mole of the compound. The freezing point can be calculated using the formula – ΔTf = i * X, where i is the number of ions formed and X is the concentration.
Does anything freeze above 0?
Almost any solid abject around you would fit this description – an aluminum soda can, a candle or a piece of chocolate all have freezing points above 0°C. Keep in mind that a material’s freezing point is the same as its melting point.
ALEKS: Predicting relative boiling point elevations and freezing point depressions
Images related to the topicALEKS: Predicting relative boiling point elevations and freezing point depressions

What do you mean by highest freezing point?
A substance which, in the aqueous solution, furnishes the minimum number of particles will lower the freezing point to the minimum. Therefore, this solution will have the maximum freezing point.
Can water freeze above 0 degrees?
Ice, at least at atmospheric pressure, cannot form above the melting point of water (0 Celsius). The phenomenon of water freezing on objects like the ground, parked cars, motorbikes etc, is due to thermal inertia.
Related searches to which of the following liquids will have the highest freezing point?
- which of the following aqueous solutions will have the highest boiling point
- which of the following aqueous solutions will have the highest boiling point?
- of the following a 0 1 m aqueous solution of will have the highest freezing point
- which of the following 0.2 m aqueous solution will have the highest freezing point
- of the following, a 0.1 m aqueous solution of will have the highest freezing point
- which of the following aqueous liquids will have the lowest freezing point
- which of the following has the lowest freezing point quizlet
- which of the following liquids will have the lowest freezing point
- which of the following liquids will have the highest freezing point pure h2o
- which of the following liquids will have the highest freezing point quizlet
- which of the following 0 2 m aqueous solution will have the highest freezing point
- which of the following liquids will have the lowest freezing point chegg
Information related to the topic which of the following liquids will have the highest freezing point?
Here are the search results of the thread which of the following liquids will have the highest freezing point? from Bing. You can read more if you want.
You have just come across an article on the topic which of the following liquids will have the highest freezing point?. If you found this article useful, please share it. Thank you very much.