Are you looking for an answer to the topic “which of the following is insoluble in water labster“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Keep Reading

Which of the following is not soluble in water quizlet?
Hydrogen (H2) is not soluble in water this is because hydrogen is a non-polar molecule whereas water is a polar molecule. There is a principle that ‘like dissolves like’ since hydrogen and water are two different types of substances, hence H2 is not soluble in water.
What does KCl Labster mean?
Copper wire. Our control KCl showed highly solubility in water. What does KCl actually stand for? Potassium chloride.
E12-TITRATION: NEUTRALIZE AN ACID LAKE CONTAMINATION
Images related to the topicE12-TITRATION: NEUTRALIZE AN ACID LAKE CONTAMINATION

What type of compound is substance 2?
Substance 1 is a covalent compound and Substance 2 is an ionic compound.
Why does the correct Lewis structure of CO2 involve a double bond between each of the oxygen atoms and the carbon atom quizlet?
Answer: Carbon dioxide has a total of 16 valence electrons, 4 from carbon and 6 from each of the two oxygen atoms. In order to give the central carbon atom a complete octet, you need to form two double bonds with the two oxygen atoms. These bonds will account for 8 of the 16 valence electrons the molecule has.
Which of following is insoluble in water?
The correct answer is Calcium Carbonate.
What is not soluble in water?
We call substances that dissolve in water soluble. Sugar and salt are examples of soluble substances. Substances that do not dissolve in water are called insoluble. Sand and flour are examples of insoluble substances.
What happens when ionic compounds dissolve in water Labster?
Most ionic compounds show a high solubility, meaning they can be dissolved in water. They dissociate into their ions when dissolved into water. The charged ions interact with the partially negative oxygen and partially positive hydrogen in H2O.
See some more details on the topic which of the following is insoluble in water labster here:
OL Lab 4-Ionic and Covalent Bonds.docx – Course Hero
Copper wire is insoluble to water because it does not dissolve in water. It is a solid material that will still remain its original composition when put in …
Ionic and Covalent Bonds Virtual Lab – Labster
Test solubility and conductivity. Atoms can interact in many different … You will explore how these properties differ in ionic and covalent compounds.
6uil
What happens when ionic compounds dissolve in water quizlet labster. … the bonds holding these ions together, then the ionic compound dissolves in water.
What Happens When an Ionic Compound Dissolves in Water?
Water will dissolve table salt, which is an ionic compound. … These ions are so vital to metabolism that they must be replenished when the …
What does KCl stand for?
| Acronym | Definition |
|---|---|
| KCL | King’s College London |
| KCL | Potassium Chloride |
| KCL | Kyoto Common Lisp |
| KCL | Kirchoff’s Current Law |
What are covalent bonds Labster?
Covalent bonds are interatomic linkages that result from the sharing of an electron pair between two atoms.
Which type of compounds are soluble in water?
| Which ions are soluble? | |
|---|---|
| Ammonium ions NH4+ | |
| Nitrates, acetates, chlorates, and perchlorate NO3–, C2H3O2–, ClO3–, ClO4– | |
| Binary compounds of halogens (chloride, bromide, iodide, etc.) with metals Cl–, Br–, I–, etc. | Fluoride Silver, lead, and mercury F–, Ag+, Pb2+*, and Hg2+ *Lead halides are soluble in hot water. |
Do electrons become ions?
By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change.
Which substances were ionic?
- LiF – Lithium Fluoride.
- LiCl – Lithium Chloride.
- LiBr – Lithium Bromide.
- LiI – Lithium Iodide.
- NaF – Sodium Fluoride.
- NaCl – Sodium Chloride.
- NaBr – Sodium Bromide.
- NaI – Sodium Iodide.
How can testing soil help to increase the productivity of farmland?
How can testing soil help to increase the productivity of farmland? Testing reveals if soil contains the right chemicals to grow a crop and suggests way to improve soil.
What happens when ionic compounds dissolve in water quizlet?
when an ionic compound dissolves in water, the positive ends of the water molecules are attracted to the anions and the negative ends are attracted to the cations.
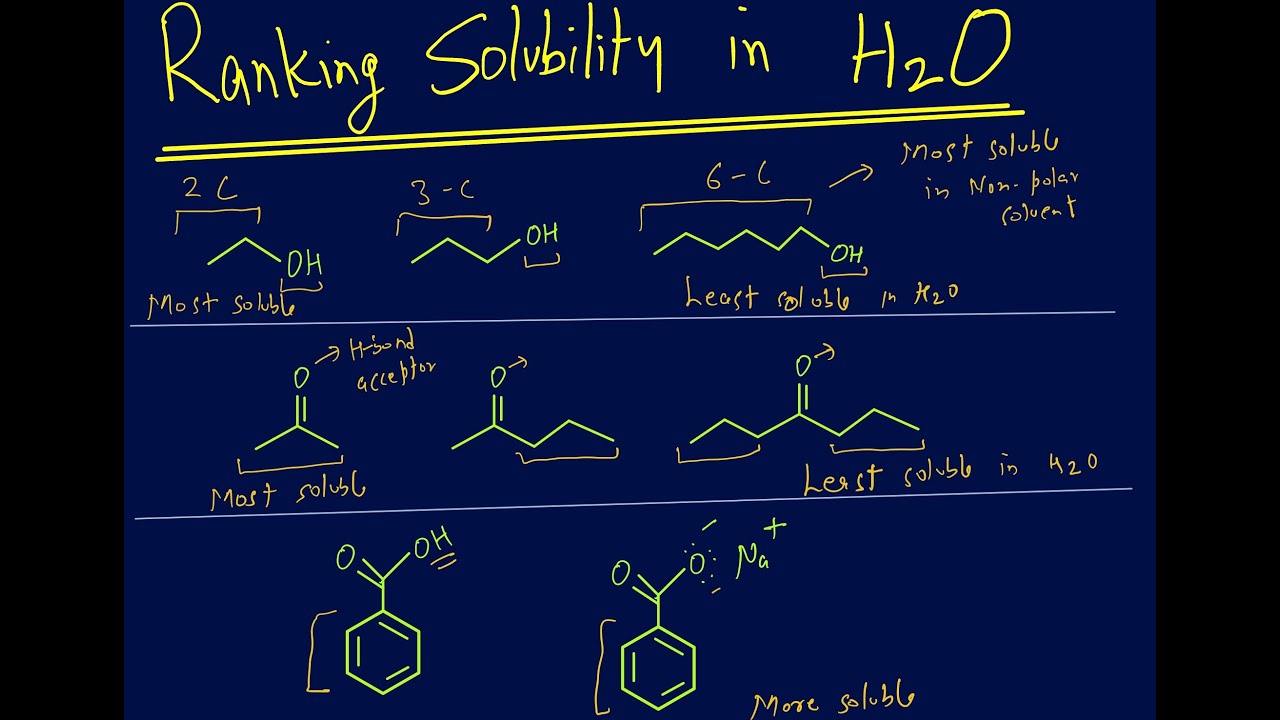
Ranking solubility of organic compounds in water based on IMF
Images related to the topicRanking solubility of organic compounds in water based on IMF
Why are the melting points of ionic compounds higher than covalent compounds quizlet?
Why? Ionic compounds have high melting points and boiling points because the electrostatic forces of attraction between oppositely charged ions are strong and large amount of energy is require to separate the ions.
Which of the following is water insoluble Mcq?
3. Which of the following is water insoluble? Explanation: C17H35 is hydrophobic in nature and hence it is water insoluble.
Which of the following is insoluble in water class 6?
Methane and nitrogen are two gases that are insoluble in water.
Is sugar is insoluble in water?
Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules.
What are the 10 examples of insoluble?
- Sand is insoluble in water.
- Chalk is insoluble in water.
- Benzene is insoluble in water.
- Oil is insoluble in water.
What are insoluble substances?
Definition: An insoluble substance is a substance (solid) that will not dissolve in a solvent even after mixing (eg; sand and water).
Why is sand insoluble in water?
Dissolution is not a chemical change. However, not all substances will dissolve in water. Salts will dissolve, the covalent bond of water “rips” the ionic bonds of the salts. Sand will not dissolve in water because the “bond” of water is not strong enough to dissolve the sand.
Are metallic bonds soluble in water?
Solubility and compound formation
Metals are insoluble in water or organic solvents, unless they undergo a reaction with them. Typically, this is an oxidation reaction that robs the metal atoms of their itinerant electrons, destroying the metallic bonding.
Are ionic compounds soluble in water?
Most ionic compounds are soluble in water. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into the water and ionic compounds are soluble in water.
Are covalent bonds soluble in water?
1 Answer. Covalent bonds do not dissolve in water, but some covalent compounds do. Covalent molecules are attracted to each other by various intermolecular forces such as H-bonds, dipole-dipole forces, and London dispersion forces. Water molecules are attracted to each other by strong H-bonds.
Which of the following is most soluble in water quizlet?
NaCl is the most soluble because it is a salt.
Is ccl4 soluble in water?
Substitution vs Elimination: Predict the outcome – Labster Simulation Guide
Images related to the topicSubstitution vs Elimination: Predict the outcome – Labster Simulation Guide

Is caco3 soluble in water?
Calcium carbonate has a very low solubility in pure water (15 mg/L at 25°C), but in rainwater saturated with carbon dioxide, its solubility increases due to the formation of more soluble calcium bicarbonate. Calcium carbonate is unusual in that its solubility increases as the temperature of the water decreases.
Is k2so4 soluble in water?
Related searches to which of the following is insoluble in water labster
- what type of compound is substance 2 most likely to be
- which of the three chemicals substance 1
- when k and cl form an ionic bond labster
- which of following is insoluble in water
- based on the melting points of both substances in comparison with the table whats your guess
- which of the three chemicals (substance 1)
- which of the three chemicals substance 1 substance 2 and kcl are conductive in water
- which of the following is insoluble in water quizlet
- which of the three chemicals are conductive in water labster
- when k+ and cl- form an ionic bond labster
- looking at the table, which rule concerning melting behavior can you formulate
- which of the following is insoluble in water labster quizlet
- looking at the table which rule concerning melting behavior can you formulate
- based on the melting points of both substances in comparison with the table what’s your guess
Information related to the topic which of the following is insoluble in water labster
Here are the search results of the thread which of the following is insoluble in water labster from Bing. You can read more if you want.
You have just come across an article on the topic which of the following is insoluble in water labster. If you found this article useful, please share it. Thank you very much.