What is an example of a bidentate ligand?
Let’s look at two popular examples:
Ethylenediamine (en): This ligand has two nitrogen atoms that can form bonds with a metal. It’s like a little bridge connecting the metal to two different parts of the molecule.
Oxalate ion (ox): This ligand has two oxygen atoms that can bond with a metal. It’s a bit like a tiny pincer grabbing onto the metal at two points.
These bidentate ligands are important in chemistry because they can form stable complexes with metals. This stability comes from the fact that the ligand is held onto the metal at two points, making it less likely to fall off. This property is useful in many applications, such as in catalysis and in the synthesis of new materials.
Let’s dive deeper into these examples to get a better understanding of their structure and bonding.
Ethylenediamine (en) is a simple organic molecule with the formula NH2CH2CH2NH2. It contains two amine groups (-NH2) that act as donor atoms. These nitrogen atoms have lone pairs of electrons that can form coordinate covalent bonds with metal ions. When ethylenediamine binds to a metal, it forms a chelate ring – a ring structure that includes the metal and the ligand. This ring formation adds stability to the complex.
Oxalate ion (ox) has the formula C2O4^2-. It contains two carboxylate groups (-COO-) that act as donor atoms. The oxygen atoms in these groups have lone pairs of electrons that can form coordinate covalent bonds with metal ions. Like ethylenediamine, oxalate forms a chelate ring when it binds to a metal, enhancing the stability of the complex.
The ability of these bidentate ligands to form chelate rings is crucial in many chemical processes. For example, in catalysis, bidentate ligands can help stabilize transition states and promote specific reactions. In material science, bidentate ligands are used to create new materials with unique properties, such as luminescence and conductivity.
So, next time you see a bidentate ligand, remember that it’s more than just a molecule – it’s a versatile tool that can be used to create stable complexes and drive important chemical reactions.
Is C204 a bidentate ligand?
Think of it like this: A monodentate ligand has only one tooth, so it can only grab onto the metal ion once. But a bidentate ligand has two teeth, so it can grab onto the metal ion twice.
Here’s a breakdown of how oxalate works:
Structure:Oxalate has a central carbon chain (C2) with two oxygen atoms on each end.
Bonding: The two oxygen atoms on each end of the oxalate molecule are capable of forming coordinate covalent bonds with the metal ion. This is because they have lone pairs of electrons they can donate to the metal.
Coordination: These two bonds form a chelate ring, where the metal ion is trapped between the two oxygen atoms of oxalate.
Chelation, the process of forming a chelate ring, is important because it strengthens the bond between the metal ion and the ligand. It also makes the complex more stable.
You can see why oxalate is a bidentate ligand. It’s like a tiny pair of pincers, ready to grab onto a metal ion and form a strong, stable bond.
Is co3 2 a bidentate ligand?
A monodentate ligand is a molecule that can bind to a metal ion at only one point. In the case of CO3 2- acting as a monodentate ligand, it will bind to the metal ion through one of its oxygen atoms.
A bidentate ligand, on the other hand, can bind to a metal ion at two points. When CO3 2- acts as a bidentate ligand, it will bind to the metal ion through two of its oxygen atoms.
Here’s a visual representation:
Monodentate Binding:
“`
O
/ \
M -O
\ /
O
“`
Bidentate Binding:
“`
O
/ \
M -O
| |
O
“`
In both cases, the metal ion (M) is represented by the symbol “M”.
The carbonate ion can also act as a bridge between two metal ions, which means it can bind to two metal ions simultaneously. This is possible because it has two oxygen atoms that can form coordinate bonds.
Now, let’s delve deeper into why CO3 2- can be both monodentate and bidentate. The carbonate ion has a trigonal planar geometry, with three oxygen atoms surrounding a central carbon atom. Each oxygen atom has a lone pair of electrons, making it capable of donating electrons to a metal ion to form a coordinate bond.
The ability of CO3 2- to act as either a monodentate or bidentate ligand depends on several factors, including the nature of the metal ion, the solvent, and the temperature. However, its versatility in binding makes it a valuable ligand in various coordination compounds.
For example, CO3 2- is commonly found as a ligand in transition metal complexes, where it can exhibit both monodentate and bidentate behavior. These complexes are important in various applications, including catalysis, medicine, and materials science.
Understanding the bonding capabilities of ligands like CO3 2- is crucial in comprehending the structure, properties, and reactivity of coordination compounds. It helps us unravel the intricate world of inorganic chemistry, paving the way for new discoveries and advancements.
What is an example of a bidentate chelating ligand?
One great example of a bidentate chelating ligand is ethylenediamine, which has the formula NH2CH2CH2NH2. This ligand has two nitrogen atoms that can donate electrons to the metal ion, forming a stable complex.
Here are some more examples of bidentate ligands:
β-diketones (coordinating atoms O,O)
acylpyrazolones (coordinating atoms O,N)
bipyridyl (coordinating atoms N,N)
functionalized derivatives of chalcones (coordinating atoms O,O)
Schiff bases (coordinating atoms N,O; N,N; N,S)
sulfonamides (coordinating atoms O,N)
aromatic amines
Now, let’s dive a little deeper into what makes these ligands so special. Bidentate chelating ligands create chelate rings when they bond to a metal ion. These rings are quite stable because of the chelate effect, which basically says that the more rings you have, the stronger the bond will be.
Think of it like this: if you have a single arm holding onto a ball, it’s easier to pull the ball away. But if you have two arms wrapped around it, it’s much more difficult to get it loose. The same principle applies to chelate rings! The more points of attachment a ligand has, the harder it is for the metal ion to break free.
This stability is really important in chemistry, as it can be used to influence the reactivity of metal ions. For instance, chelating ligands can be used to stabilize metal ions in solutions or to create specific chemical reactions.
So, next time you see a molecule with two potential binding sites, remember – you’re looking at a bidentate chelating ligand with the power to create stable complexes and influence the chemistry of metal ions!
Is EDTA a bidentate ligand?
Think of EDTA as a molecular octopus with six arms. Each arm is a donor atom that can grab onto a metal ion. These donor atoms are four oxygen atoms from the carboxyl groups (-COO-) and two nitrogen atoms from the amine groups (-NH2) within the EDTA molecule.
Here’s why EDTA’s structure makes it so effective:
Flexibility: EDTA’s structure allows it to wrap around metal ions and form stable complexes. This flexibility is crucial for its chelating abilities.
Multiple Coordination Sites: With six donor atoms, EDTA can form strong bonds with a wide range of metal ions. This makes it a versatile chelator.
So, while EDTA might appear complicated at first glance, its ability to form six coordinate bonds with a metal ion makes it a potent hexadentate ligand, not a bidentate one.
Which of the following ligands are bidentate?
Let’s break down what that means:
Ligands: These are molecules or ions that bind to a central metal atom or ion to form a coordination complex.
Bidentate: A bidentate ligand forms two bonds with a central metal atom. Imagine it like a pair of arms, each arm grasping the metal ion.
Oxalate ion: This ion has a structure where two carboxylate groups (COO⁻) are attached to a central carbon atom. Each carboxylate group has an oxygen atom with a lone pair of electrons that can donate to a metal ion.
The ability of the oxalate ion to donate two pairs of electrons makes it an excellent chelating agent. Chelating agents are ligands that can form multiple bonds with a single metal ion, creating a ring-like structure. This ring-like structure enhances the stability of the coordination complex. The oxalate ion’s chelating ability has important applications in various fields, such as:
Analytical chemistry: The oxalate ion can be used to determine the concentration of various metal ions, since it forms stable complexes with them.
Biochemistry: Oxalates play a role in the body’s metabolism, particularly in the synthesis of oxalic acid, which is a natural component of many plants.
Industrial chemistry: Oxalates are used in various industrial processes, including the production of dyes, pigments, and pharmaceuticals.
The oxalate ion is a good example of how a ligand’s structure can influence its coordination chemistry and its applications in different fields. By understanding the concept of bidentate ligands and their chelating abilities, we can gain a deeper appreciation for the fascinating world of coordination chemistry and its impact on our daily lives.
Is NO2 a bidentate ligand?
You’re right to ask this question! NO2 has two potential donor atoms – the nitrogen and one of the oxygen atoms. This makes it seem like it could act as a bidentate ligand, forming a ring structure with a metal ion.
However, in most cases, NO2 acts as a monodentate ligand. This means it only uses one of its donor atoms to bond to the metal ion.
Here’s why:
Steric hindrance: The two oxygen atoms in NO2 are quite close together, making it difficult for both of them to bind to the metal ion simultaneously. This crowding effect, called steric hindrance, makes it energetically unfavorable for NO2 to act as a bidentate ligand.
Electronic factors: The nitrogen atom in NO2 is more electronegative than the oxygen atoms, making it a better donor atom. This means the nitrogen atom is more likely to form a bond with the metal ion, and the oxygen atoms are less likely to get involved in bonding.
But there’s a catch! NO2 can sometimes act as a bidentate ligand under specific conditions. This typically occurs when the metal ion has a high positive charge and a small ionic radius, which can overcome the steric and electronic limitations.
For example, in some complexes with transition metals like cobalt, NO2 can act as a bidentate ligand. In these cases, the metal ion’s strong attraction to the oxygen atoms overcomes the steric and electronic factors, allowing for a bidentate coordination.
So, while NO2 generally acts as a monodentate ligand, it can be influenced by the specific metal ion and surrounding conditions to exhibit bidentate behavior.
Is NH3 a bidentate ligand?
NH3 has a central nitrogen atom with a lone pair of electrons. This lone pair can be donated to a metal ion, forming a coordinate covalent bond. Since NH3 only has one atom that can donate its electron pair, it can only bind to a metal ion at one point, making it a monodentate ligand.
Think of it this way: a monodentate ligand is like a single-pronged fork, while a bidentate ligand is like a two-pronged fork. The single-pronged fork can only grab onto one thing at a time, just like NH3 can only bind to a metal ion at one point.
Bidentate ligands, on the other hand, have two atoms with lone pairs that can donate electrons. These ligands can bind to a metal ion at two points, forming a chelate complex. A chelate complex is a ring-like structure formed when a ligand binds to a metal ion at two or more points.
A good example of a bidentate ligand is ethylenediamine (en), with the formula H2NCH2CH2NH2. It has two nitrogen atoms, each with a lone pair, that can donate electrons to a metal ion. When ethylenediamine binds to a metal ion, it forms a five-membered ring, which is a stable structure.
In contrast, NH3 can’t form a chelate complex because it only has one nitrogen atom with a lone pair. It can only bind to a metal ion at one point, making it a monodentate ligand.
Is cl2 a bidentate ligand?
A bidentate ligand needs to have at least two spots where it can bond to a central metal atom. Cl2, or chlorine gas, only has one lone pair of electrons available for bonding. This means it can only attach to a metal atom at a single point, making it a monodentate ligand.
Think of it like this: Imagine a metal atom as a dance floor, and the ligands as dancers. A bidentate ligand is like a tango dancer, able to hold onto the metal atom with two hands (two bonding sites). But Cl2 is more like a solo dancer, only able to hold on with one hand (one bonding site).
You might be wondering why Cl2 only has one bonding site. It’s all about the structure of the molecule. Each chlorine atom has seven valence electrons, and they share one electron with each other to form a single covalent bond. This leaves one lone pair of electrons on each chlorine atom available for bonding.
Now, you might be thinking, “But wait, doesn’t Cl2 have two lone pairs, one on each chlorine atom?” It’s true that each chlorine atom does have a lone pair, but they are localized on the individual chlorine atoms. They can’t both simultaneously bond to the same metal atom to make a bidentate ligand.
Let’s take an example: consider the [Co(NH3)4Cl2] complex. In this case, the Cl atoms are acting as monodentate ligands, each donating one lone pair of electrons to the central cobalt atom.
So, while Cl2 might look like it could be a bidentate ligand, it simply doesn’t have the right structure or the necessary bonding sites to make it happen.
See more here: Is C204 A Bidentate Ligand? | Which Of The Following Is A Bidentate Ligand
See more new information: barkmanoil.com
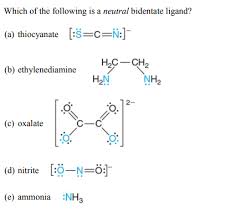
Which Of The Following Is A Bidentate Ligand?
So, what exactly is a bidentate ligand? Well, picture it like this: imagine a ligand as a little molecule that wants to snuggle up to a metal ion to form a coordination complex. Now, a bidentate ligand is a special type of ligand that’s got a bit more going on—it has twodonor atoms that can bond to the metal ion. That means it can hold on to the metal ion with two strong arms, or coordination sites.
Think of it like a two-armed hug. A monodentate ligand, on the other hand, is like a one-armed hug – it can only bond with the metal ion at one site.
Examples of Bidentate Ligands
Let’s look at some real-world examples to make this clearer. Ethylenediamine, often abbreviated as en, is a classic example of a bidentate ligand. Its structure is simple: two nitrogen atoms, each with a lone pair of electrons, connected by a two-carbon chain. Each nitrogen atom can donate its lone pair to the metal ion, creating a stable coordination complex.
Another common example is oxalate, which is a dicarboxylate anion. It has two oxygen atoms with lone pairs, and each oxygen can bond to the metal ion.
Why are Bidentate Ligands Special?
You might be wondering why all this fuss about bidentate ligands. Why are they so important? Well, there are a few key reasons:
1. Enhanced Stability:Bidentate ligands can form much more stable coordination complexes than monodentate ligands. This is because the metal ion is held in place by two bonds, making it much harder to detach.
2. Chelate Effect: The enhanced stability of coordination complexes formed by bidentate ligands is known as the chelate effect. It’s like a ligand is giving the metal ion a big, warm hug that it doesn’t want to let go of.
3. Controlled Reactivity: The formation of coordination complexes with bidentate ligands can influence the reactivity of the metal ion. This is because the ligands can affect the electron density around the metal center.
Identifying Bidentate Ligands
To identify whether a ligand is bidentate, look for these key features:
Two Donor Atoms: The ligand must have at least two atoms capable of donating a lone pair of electrons to the metal ion.
Ability to Form Two Bonds: The ligand should be able to form two coordinate covalent bonds with the metal ion.
Appropriate Geometry: The ligand needs to have the right geometry to allow for the formation of two bonds with the metal ion.
Some Important Things to Remember:
Ligands can be monodentate, bidentate, tridentate, tetradentate, or even polydentate. A tridentate ligand has three donor atoms, a tetradentate ligand has four, and so on.
Chelate effect is an important concept in coordination chemistry, and it has significant applications in many areas.
FAQs
Q: What are some examples of bidentate ligands in coordination complexes?
A: Besides ethylenediamine (en) and oxalate, other common examples include:
Carbonate ion (CO3^2-)
Oxalato ion (C2O4^2-)
Glycinate ion (NH2CH2COO-)
Acetylacetonate ion (acac-)
Q: How can I tell if a ligand is bidentate or not?
A: Look for these clues:
Donor Atoms: Are there two or more atoms that have lone pairs of electrons?
Geometry: Can the ligand arrange itself to form two bonds with the metal ion?
Stability: Does the complex formed by the ligand exhibit enhanced stability compared to complexes with monodentate ligands?
Q: What are some real-world applications of bidentate ligands?
A: Bidentate ligands play a vital role in various applications, including:
Catalysis: Bidentate ligands can be used to create catalysts with specific properties.
Medicine: Certain bidentate ligands can bind to metal ions in the body, helping to treat heavy metal poisoning or deliver drugs to specific tissues.
Environmental Remediation: Bidentate ligands can be used to remove harmful metal ions from wastewater or contaminated soil.
Q: What is the difference between a bidentate ligand and a monodentate ligand?
A: A bidentate ligand can form two bonds with a metal ion, while a monodentate ligand can only form one bond. Think of it like a two-armed hug versus a one-armed hug.
Q: Why is the chelate effect important?
A: The chelate effect explains the increased stability of coordination complexes formed by bidentate ligands. This enhanced stability has many applications in different fields.
I hope this information is helpful! If you have any more questions about bidentate ligands or coordination chemistry in general, feel free to ask!
24.2: Ligands – Chemistry LibreTexts
Bidentate ligands. Bidentate ligands have two lone pairs, both of which can bond to the central metal ion. The two commonly used examples are 1,2-diaminoethane (old name: Chemistry LibreTexts
Which of the following ligands is expected to be bidentate? – BYJU’S
Question. Which of the following ligands is expected to be bidentate? A. CH3N H2. B. CH3C≡N. Br. D. C2O2− 4. Solution. The correct option is D C2O2− 4. When a ligand BYJU’S
Ligands – Chemistry LibreTexts
Bidentate Ligands. Bidentate ligands have two donor atoms which allow them to bind to a central metal atom or ion at two points. Common examples of Chemistry LibreTexts
3.1.1: Chelating Ligands – Chemistry LibreTexts
Many factors can influence the bite angle, including structural features of the bidentate ligand itself, the metal, and other ligands bound to the metal. However, a particular ligand will usually have a normal range of bite Chemistry LibreTexts
Structures With Bidentate Ligands – Purdue University
Bidentate ligands are Lewis bases that donate two pairs (“bi”) of electrons to a metal atom. Bidentate ligands are often referred to as chelating ligands (“chelate” is derived Purdue Chemistry
Coordination Compounds Help Page – Purdue University
Structures With Bidentate Ligands. Bidentate ligands are Lewis bases that donate two pairs (“bi”) of electrons to a metal atom. Bidentate ligands are often referred to as Purdue Chemistry
Which of the following ligands is expected to be bidentate? – Toppr
Question. Which of the following ligands is expected to be bidentate? Br. C2O2− 4. CH 3N H 2. CH 3C ≡ N. A. Br. CH3N H2. CH3C ≡N. D. C2O2− 4. Solution. Verified by Toppr. Toppr
Which of the following is/are bidentate ligand? – Careers360
Bidentate ligands are ligands with two donor atoms which can link with the central metal ion through two co-ordinate bonds. Some examples include ethylenediamine(en), Careers360
Which Of The Following Is A Bidentate Ligand ?
Which Of The Following Is A Bidentate Ligand? | 12 | Coordination Compounds | Chemistry | Grb Pu…
Which Of The Following Is An Unsymmetrical Bidentate Ligand? | 12 | Coordination Compounds | Ch…
Which Of The Following Ligands Is Expected To Be Bidentate? (A) Ch_3Nh_2 (B) Ch_3C≡N (C) Br (D) C…
Which Of Th Efollowing Is A Bivalent And Bidentate Ligand?
Which Of The Following Is A Bidentate Ligand? | Class 12 | Coordination Compounds | Chemistry | …
Which Of The Following Acts As A Bidentate Ligand In Complex Formation?
Select Bidentate Or Didentate Ligand From The Following . \\( P \\) (…
Link to this article: which of the following is a bidentate ligand.
See more articles in the same category here: https://barkmanoil.com/bio