Are you looking for an answer to the topic “which elements have complete outer shells“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Group 0 elements – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn) – have full outer shells. (Group 0 is sometimes called Group 8 – all the elements in the group have eight electrons in their outer shell, except for helium which only has two).Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.A full outer shell is known as the noble gas configuration where the outer shell of an atom is energetically stable and contains 8 outer electrons.
Which elements had complete outer shells?
Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.
What are complete outer shells?
A full outer shell is known as the noble gas configuration where the outer shell of an atom is energetically stable and contains 8 outer electrons.
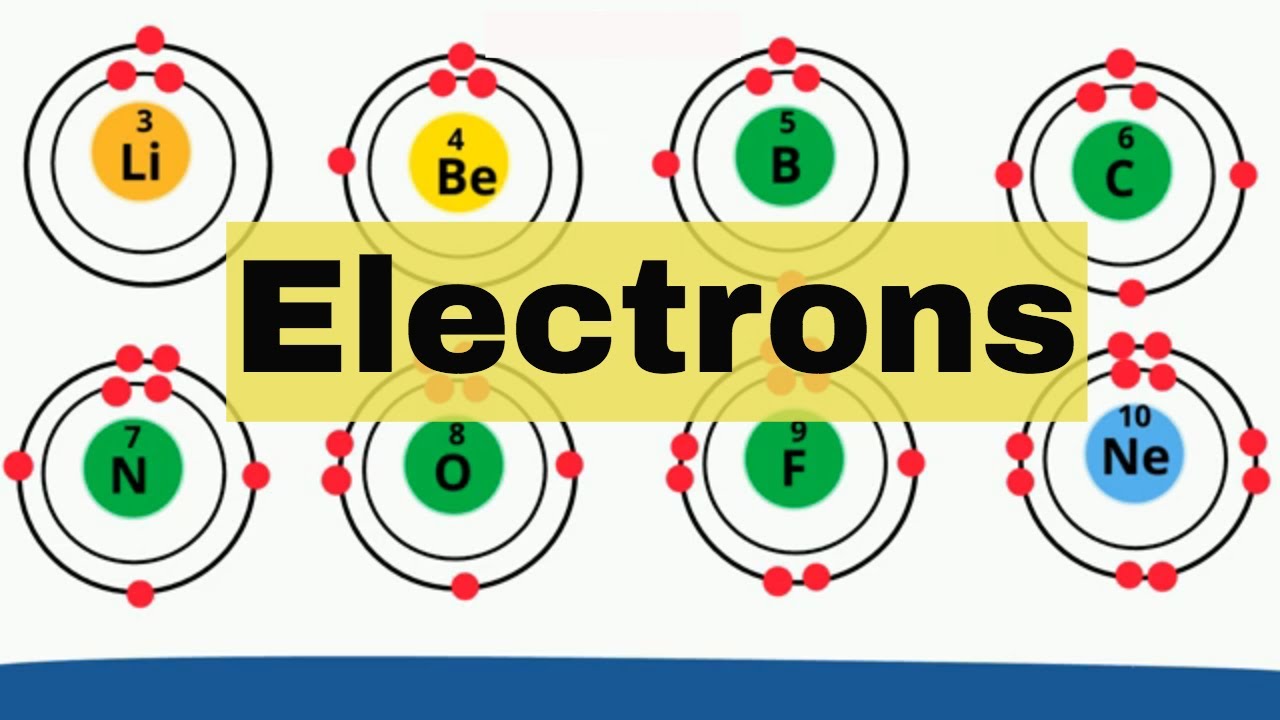
Electron shells Elements 1-18
Images related to the topicElectron shells Elements 1-18

Which element has complete outer most shell?
The elements which have their outermost shell complete are called noble gases. This group has eight electrons in its outermost orbit (except helium which has two electrons). As a result, they have a stable arrangement. Group 18 elements are gases with a low chemical reactivity, meaning they don’t form many compounds.
Which group has a full outer shell of electrons?
Group 0 elements – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn) – have full outer shells. (Group 0 is sometimes called Group 8 – all the elements in the group have eight electrons in their outer shell, except for helium which only has two).
Does argon have a complete outer shell?
Just like neon (Ne) and helium (He), argon (Ar) usually floats around all by itself. It is non-reactive because the shells are full. Argon has three electron shells. The third shell is filled with eight electrons.
Does neon have a complete outer shell?
Similarly, neon has a complete outer 2n shell containing eight electrons. These electron configurations make helium and neon very stable.
Which element has a complete valence electron shell?
There are two elements that have complete valence electron shells. These elements are helium and neon.
See some more details on the topic which elements have complete outer shells here:
Chemical Bonding | Biology for Non-Majors I
Helium (He), neon (Ne), and argon (Ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. · Hydrogen (H), lithium (Li), …
2.1E: Electron Shells and the Bohr Model – Biology LibreTexts
Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron …
The periodic table, electron shells, and orbitals (article) – Khan …
Although argon does not technically have a full outer shell, since the 3n shell can hold up to eighteen electrons, it is stable like neon and helium because it …
Which Elements Had Complete Outer Shells Give The Name …
Helium (He), neon (Ne), and argon (Ar), as group 18 elements, have outer electron shells which are full or fulfill the octet rule.
Does xenon have a full electron shell?
Answer and Explanation: Xenon has eight valence electrons, which are the electrons in its outer shell. This means that the outer shell is full, making xenon a stable element….
Which elements have their outermost shell incomplete?
- (i) Outermost shell complete – Noble gases.
- (ii) Outermost shell incomplete – Representative elements.
- (iii) two outermost shell incomplete – Transition elements.
- (iv)one electron short of octet – Halogens.
- (v) two electrons in the outermost orbit – Alkaline Earth metals.
How many shells does lithium have?
…
Electron shells.
| Energy shell | Maximum number of electrons |
|---|---|
| Second | 8 |
| Third | 8 |
Which of the following elements contain only two electrons in the outermost shell?
The correct answer is Magnesium. The outermost shell of a Magnesium element has two electrons.
What element has a full outer shell and has 6 energy levels?
| Atomic Number | Element | Energy Levels or “shells” |
|---|---|---|
| 6 | Carbon (C) | 4 |
| 7 | Nitrogen (N) | 5 |
| 8 | Oxygen (O) | 6 |
| 9 | Fluorine (F) | 7 |
Bonding (Ionic, Covalent Metallic) – GCSE Chemistry
Images related to the topicBonding (Ionic, Covalent Metallic) – GCSE Chemistry

Which of the following elements has a single electron in its outermost shell?
(a) Lithium (Li), sodium (Na), and potassium (K) have a single electron in their outermost shells.
Which of the following elements has the most valence electrons in its outermost shell?
All noble gases have the maximum number of valence electrons in their outermost shell. This means they do not seek to gain or lose any electrons and are unreactive. What do the periods (rows) and groups (columns) on the periodic table tell us?
How many shells does xenon have?
| Xenon | |
|---|---|
| Period | period 5 |
| Block | p-block |
| Electron configuration | [Kr] 4d10 5s2 5p6 |
| Electrons per shell | 2, 8, 18, 18, 8 |
How many shells does potassium have?
The electron configuration of potassium-ion shows that potassium ion have three shells and the last shell has eight electrons. This electron configuration shows that the potassium atom has acquired the electron configuration of argon.
How many shells does hydrogen have?
Hydrogen has 1 outer shell which contains 1 electron.
How many shells does boron have?
The boron atom has only six electrons in its outer shell, leading to an electron deficiency. This molecule has 12 valence shell electrons; 3 each from the B atoms, and 1 each from the six H atoms.
How many shells does sulfur have?
The electron configuration of sulfur ion shows that sulfur ion has three shells and the last shell has eight electrons.
How many shells does magnesium have?
Magnesium has a total of 12 electrons – 2 in the innermost shell, 8 in the second shell, and two electrons in its valence shell (third shell). Magnesium acquires a full octet by losing 2 electrons and emptying out its outermost shell.
Which elements had complete outer shells give the name and symbols of each?
Helium (He), neon (Ne), and argon (Ar), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. This makes them highly stable as single atoms. Because of their non-reactivity, they are called the inert gases or noble gases.
Which element has a complete valence electron shell?
There are two elements that have complete valence electron shells. These elements are helium and neon.
GCSE Chemistry – Electron Arrangement #8
Images related to the topicGCSE Chemistry – Electron Arrangement #8

Does neon have a complete outer shell?
Neon is in the group of which the elements are the least reactive, the Noble Gas’s. The Noble Gas’s have a complete outer shell meaning they need no energy to have a complete outer shell.
What element has a full valence shell and is in Period 3?
The name “argon” is derived from the Greek neuter adjective ἀργόν, meaning “lazy” or “the inactive one”, as the element undergoes almost no chemical reactions. The complete octet (eight electrons) in the outer atomic shell makes argon stable and resistant to bonding with other elements.
Related searches to which elements have complete outer shells
- Why does carbon form four covalent bonds
- how many atoms are present in 123 g of magnesium cyanide
- which of these elements 2 outer shell electrons?
- chemical bonding of 02 atoms can form due to
- which elements on the periodic table have complete outer shells
- which elements have complete outer shells give the name and symbol for each
- how many atoms are present in 123 g of magnesium cyanide?
- orbital diagram of first 30 elements
- which elements have a complete outer electron shells which make them very stable
- why does carbon form four covalent bonds
- which elements have full outer shells
- arrangement of electrons in the atoms of the first 20 elements
- which of these elements 2 outer shell electrons
- which element has 84 protons?
- which element has 84 protons
- how many electrons does an atom of argon have
Information related to the topic which elements have complete outer shells
Here are the search results of the thread which elements have complete outer shells from Bing. You can read more if you want.
You have just come across an article on the topic which elements have complete outer shells. If you found this article useful, please share it. Thank you very much.