Are you looking for an answer to the topic “which atomic property is different in each isotope of an element?“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
An isotope is one of two or more forms of the same chemical element. Different isotopes of an element have the same number of protons in the nucleus, giving them the same atomic number, but a different number of neutrons giving each elemental isotope a different atomic weight.In what way do isotopes of an element differ? All the isotopes of an element have the same chemical properties because they have the same number of electrons. The isotopes of an element differ because of the number of neutrons it may contain.Isotopes are atoms with different atomic masses which have the same atomic number. The atoms of different isotopes are atoms of the same chemical element; they differ in the number of neutrons in the nucleus.
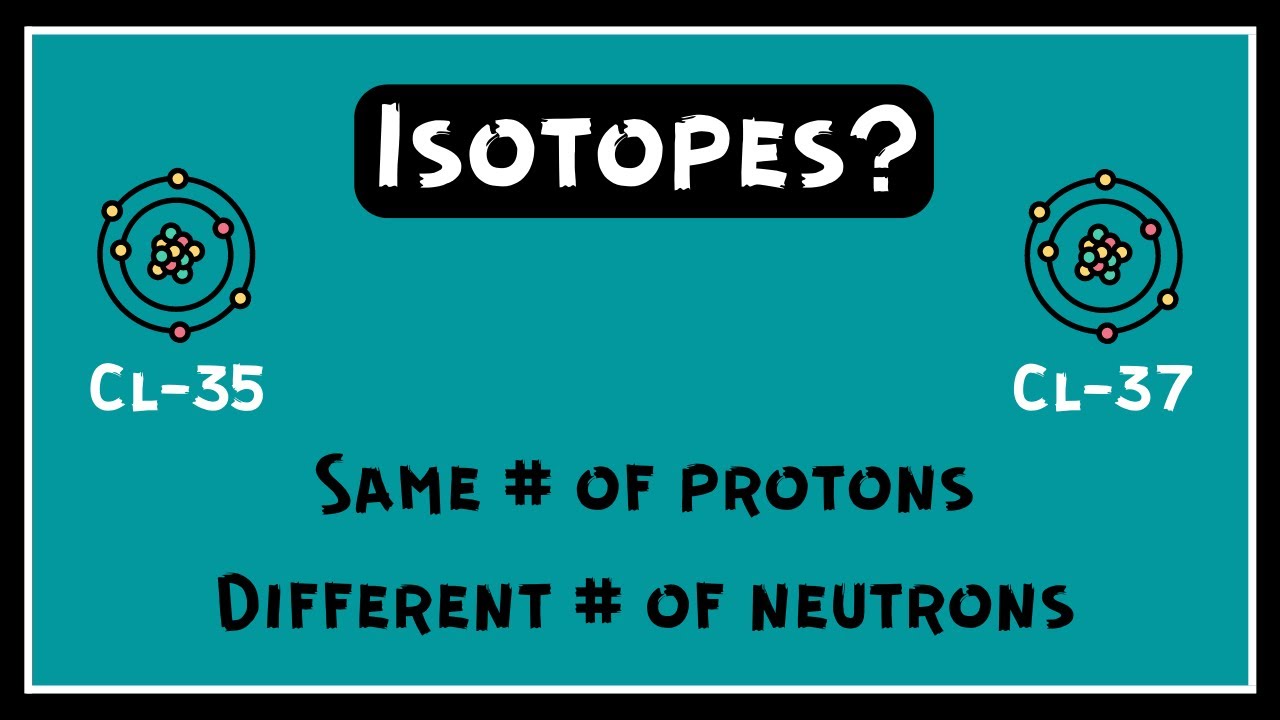
Which atomic property is different in each isotope of an element quizlet?
In what way do isotopes of an element differ? All the isotopes of an element have the same chemical properties because they have the same number of electrons. The isotopes of an element differ because of the number of neutrons it may contain.
Which atomic property is different in each isotope of an element A number of protons?
Isotopes are atoms with different atomic masses which have the same atomic number. The atoms of different isotopes are atoms of the same chemical element; they differ in the number of neutrons in the nucleus.
What is an isotope, including their chemical and physical properties | A Level Chemistry
Images related to the topicWhat is an isotope, including their chemical and physical properties | A Level Chemistry

Which properties of isotopes are different?
Answer: When it comes to the chemical properties of isotopes of a given element, they are nearly identical or identical. Chemical properties of different isotopes are nearly identical. However, physical properties of isotopes such as mass, melting or boiling point, density, and freezing point are all different.
Which property of the isotopes must be different quizlet?
The number of protons are different. Protons are what make an element that particular element. How do isotopes of a given element differ from one another? They have different mass numbers and different numbers of neutrons.
How do isotopes differ from each other?
Isotopes. An isotope is one of two or more forms of the same chemical element. Different isotopes of an element have the same number of protons in the nucleus, giving them the same atomic number, but a different number of neutrons giving each elemental isotope a different atomic weight.
Do isotopes have different chemical properties?
Different isotopes of an element generally have the same physical and chemical properties because they have the same numbers of protons and electrons.
What varies from one isotope of an element to another isotope of the same element?
Isotopes of an element share the same number of protons but have different numbers of neutrons.
See some more details on the topic which atomic property is different in each isotope of an element? here:
Which atomic property is different in each isotope of an element?
In the question “Which atomic property is different in each isotope of an element?” The correct answer is the neutron and the mass number.
4.8: Isotopes- When the Number of Neutrons Varies
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons.
Which atomic property is different in each isotope of an element?
The atomic property that is different in each isotope of an element is the atomic mass of the atom caused by changes in the number of neutrons.
ATOMS AND ISOTOPES
For many of the chemical elements there are several known isotopes. Isotopes are atoms with different atomic masses which have the same atomic number. The atoms …
What is the reason the isotopes of an element are different?
Isotope is a variant of an element where it has an equal number of protons but a different number of neutrons of the atom due to which, the isotopes have different masses. Elements with an odd atomic numbers like Hydrogen, Lithium, Sodium or Nitrogen have one or two stable isotopes.
Which of the following is a property of isotopes?
Isotopes of an element have same atomic number but different mass number. Hence, they have same number of electrons. Chemical properties are related to the number of valence electrons. Thus, isotopes have similar chemical properties.
Do isotopes have different melting points?
Different isotopes of the same element have the same number of electrons, so the nature of bonding between different isotopes of the same element should be the same, yet their melting points and boiling points vary.
What are isotopes State three properties of isotopes?
isotopes have similar atomic number and different atomic masses. they have similar chemical properties and different physical properties.
How To Find The Percent Abundance of Each Isotope – Chemistry
Images related to the topicHow To Find The Percent Abundance of Each Isotope – Chemistry
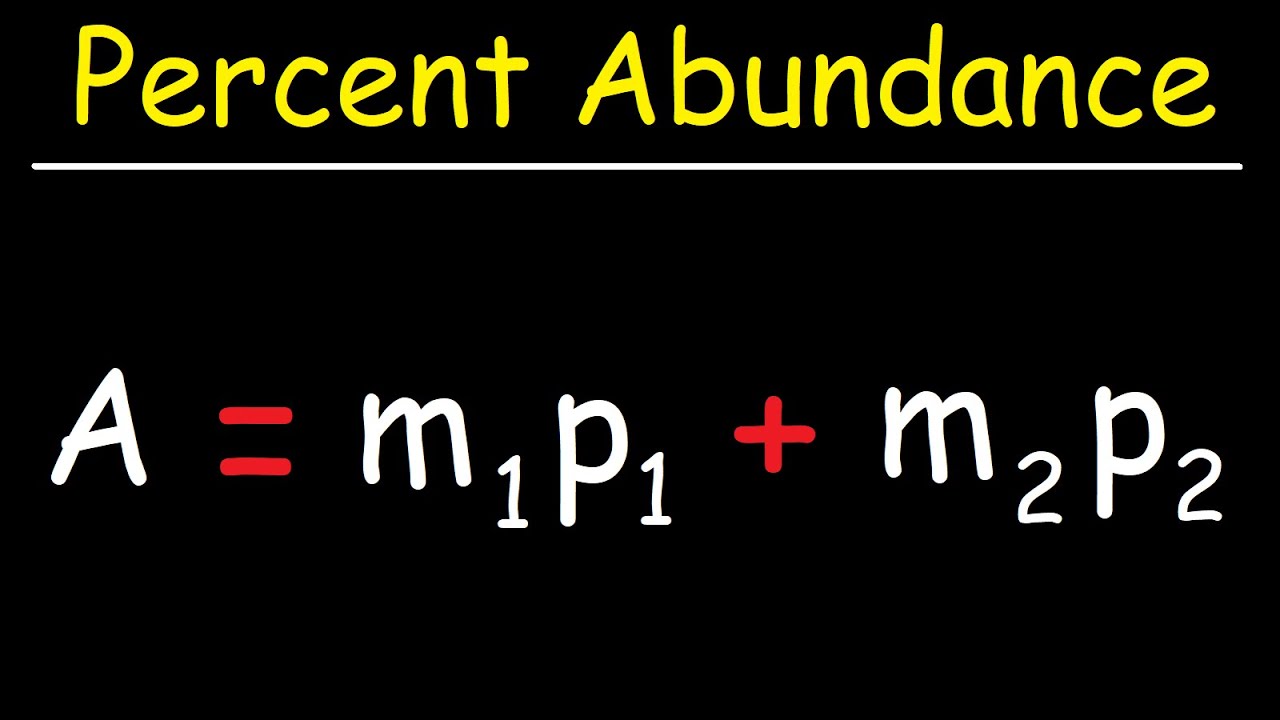
How do two isotopes of the same element differ quizlet?
Isotopes of the same element have different numbers of neutrons and therefore, different mass numbers and different atomic masses. Isotopes of the same element have the same number of portones and electrons. The electrons, not the neutrons are responsible for an element’s chemical behavior.
How do the atoms of isotopes of a particular element vary quizlet?
different atomic numbers and different atomic weights.
How do the atoms of isotopes of a particular element vary?
Isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, “Z”) but a different number of neutrons, meaning that their mass number, “A”, varies. Take hydrogen, for example.
What atomic particle differs when there are several isotopes of the same element?
Isotopes are atoms of the same element that contain an identical number of protons, but a different number of neutrons. Despite having different numbers of neutrons, isotopes of the same element have very similar physical properties.
Which of the following properties are different for neutral atoms of isotopes of the same element?
Isotopes have equal atomic number and protons, but differ in the number of neutrons.
Why do isotopes of an element have similar properties?
Isotopes are atoms of the same element. They have the same atomic number but different mass number. Number of protons and electrons in isotopes are the same . So, since the number of protons are the same and since protons determine the chemical properties of an atom, they show similar chemical properties.
Under what conditions are two atoms different isotopes of the same element?
Different numbers of neutrons and/or protons result in different nuclides. If two atoms have different numbers of protons, they are different elements. However, if two atoms have the same number of protons, but different numbers of neutrons we refer to them as isotopes.
What is an isotope of an element quizlet?
Isotopes are atoms of an element with the normal number of protons and electrons, but different numbers of neutrons. Isotopes have the same atomic number, but different mass numbers. The different isotopes of an element have identical chemical properties.
How are isotopes defined quizlet Edgenuity?
What is an isotope? Atoms of the same element with the same number of protons, but different number of neutrons.
ISOTOPES OF THE ELEMENTS
Images related to the topicISOTOPES OF THE ELEMENTS
Why do different isotopes of an element generally have the same physical and chemical properties?
Different isotopes of an element generally have the same physical and chemical properties because they have the same numbers of protons and electrons.
What is a characteristic of two isotopes of the same element?
All the Isotopes of an Element Have Identical Chemical Properties. All the Isotopes of an element have identical chemical properties because they have the same number of electrons as an atom of that element but they have different numbers of neutrons. The different number of neutrons affects the mass number.
Related searches to which atomic property is different in each isotope of an element?
- which unit is used for measuring atomic mass
- the average atomic mass of oxygen is 15 9994 amu
- which isotope is most likely to have the greatest abundance in nature?
- where are electrons of an atom found
- which isotope is most likely to have the greatest abundance in nature
- where are electrons of an atom found?
- which of the following is true for balancing equations?
- which unit is used for measuring atomic mass?
- isotopes are atoms of the same element with the same number of protons and
- the average atomic mass of oxygen is 15.9994 amu
- which atomic property is different in each isotope of an element quizlet
- which of these elements is a transition metal
- which atoms are isotopes
- which of the following is true for balancing equations
Information related to the topic which atomic property is different in each isotope of an element?
Here are the search results of the thread which atomic property is different in each isotope of an element? from Bing. You can read more if you want.
You have just come across an article on the topic which atomic property is different in each isotope of an element?. If you found this article useful, please share it. Thank you very much.
