Are you looking for an answer to the topic “what is the oxidation state of an individual carbon atom in caco3?“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Therefore, the carbon is in the +4 oxidation state.According to the structure of the carbonate ion, the two oxygen atoms attached to the carbon atom have \[ – 1\] charge over each of them. Thus, the overall charge on the carbonate ion will be \[ – 2\] and counteracting that, the calcium atom will have an oxidation state of \[ + 2\] .Therefore, the oxidation number of the carbon atom is +3 . Note that, the oxidation number of Oxygen is always −2 except in H2O2 is −1 and in OF2 is +2 .

What is the oxidation state of Ca in CaCO3?
According to the structure of the carbonate ion, the two oxygen atoms attached to the carbon atom have \[ – 1\] charge over each of them. Thus, the overall charge on the carbonate ion will be \[ – 2\] and counteracting that, the calcium atom will have an oxidation state of \[ + 2\] .
What is the oxidation state of an individual carbon atom?
Therefore, the oxidation number of the carbon atom is +3 . Note that, the oxidation number of Oxygen is always −2 except in H2O2 is −1 and in OF2 is +2 .
How to find the Oxidation Number for C in CaCO3 (Calcium carbonate)
Images related to the topicHow to find the Oxidation Number for C in CaCO3 (Calcium carbonate)

What is the oxidation state of C in CO3 2 carbonate?
The oxidation number of the carbon is +4.
What is the oxidation state of an individual carbon atom in hno3?
Therefore, Option (C) that is 5 is the correct answer. Note: Oxidation is considered to be the loss of one or more electrons by an atom.
How do you find the Valency of CaCO3?
The valency of the element is decided by the number of valence electrons. The calcium carbonate is CaCO3. The valency of calcium is +2 in the compound CaCO3 or Calcium Carbonate. Calcium atom has two free electrons in its outermost shell that is easily transferred to the CO3 or carbonate group.
What is the molecular structure of CaCO3?
Calcium carbonate is chemical compound largely used as additive in agriculture and it is also sold as a calcium supplement. Formula and structure: The calcium carbonate chemical formula is CaCO3 and its molar mass is 100.0869 g mol–1. The molecule is formed by the calcium cation Ca+2 and the carbonate anion CO3–2.
What is the oxidation number of c2?
Answer. O.N of C is + 4.
See some more details on the topic what is the oxidation state of an individual carbon atom in caco3? here:
What is the oxidation state of an individual … – Brainly.com
Answer:The oxidation state of the carbon is +4.Explanation:Calcium is in group 2 of the periodic table, therefore, its oxidation state is +2 …
What is the oxidation state of calcium in calcium … – Vedantu
According to the structure of the carbonate ion, the two oxygen atoms attached to the carbon atom have \[ – 1\] charge over each of them. Thus, the overall …
what is the oxidation state of an individual … – The Shared Web
It is +4. Actually, CaCO3 is an ionic compound, made by Ca^2+ and (CO3)^2- ions giving rise to a lattice where they regularly alternate, …
what is the oxidation state of an individual carbon atom in caco3
What is the oxidation number state of carbon C in CaCO3? + 4\] Thus, let us determine the oxidation state of the calcium atom in calcium carbonate step by step.
What is the oxidation state of the carbon atom in the compound CO2?
Also, we know that the total charge on any neutral compound is always zero and the total charge on any neutral compound will be equal to the sum of the charges on the substituent atoms. So, the oxidation number of carbon in carbon dioxide (${\text{C}}{{\text{O}}_2}$) is +4.
What is the oxidation state for each element in the polyatomic ion CO32?
Since CO32- is a polyatomic ion, the algebraic sum of all the oxidation numbers of atoms of the ion must equal the charge on the ion. Thus, the oxidation number of carbon is +4 and that of oxygen is -2.
Is CO3 positive or negative?
The substance with the chemical formula CO3 goes by the name carbonate. Carbonate is made of 1 atom of carbon and 3 atoms of oxygen and has an electric charge of −2. This negative charge means that a single ion of carbonate has 2 more electrons than protons.
Oxidation Number for CaCO3 . Oxidation state of caco3 . Calcium carbonate oxidation numbers. Caco3
Images related to the topicOxidation Number for CaCO3 . Oxidation state of caco3 . Calcium carbonate oxidation numbers. Caco3

What is the oxidation state of each individual carbon atom in c2o42 −?
Hence, the oxidation number of carbon in C2O4–2 is +3.
What is the oxidation state of an individual phosphorus atom in po43 −?
So, phosphorus has a +5 oxidation state in the phosphate anion, PO3−4 .
What is the oxidation state of an individual sulfur atom in so42 −?
The oxidation number of the sulfur atom in the SO42– ion must be +6, for example, because the sum of the oxidation numbers of the atoms in this ion must equal -2.
What is the valency of carbon in CaCO3?
Hence, there are four unpaired electrons which are capable of forming four covalent bonds to the surrounding. So, the valency of carbon in all its compounds is 4. The correct option is C. Hence the valency of carbon in carbonate is 4.
What is the valency of calcium in CaCO3 and CaCl2?
CaCO3:- In CaCO3 valency of calcium is 2 and of carbonate (CO32-) is 2 . Ca3N2:- Valency of Calcium is 2 and Valency of Nitrogen is 3. CaCl2:- Valency of Calcium is 2 and Valency of Chlorine is 1.
What electron configuration does calcium have in CaCO3?
…
General Properties of Calcium.
| Symbol | Ca |
|---|---|
| Group,Period,Block | 2,4,s |
| Electron Configuration | [Ar]4s2 |
| Valence Electrons | 2 |
| Phase (room temperature) | solid |
How many atoms are there in CaCO3?
Calcium carbonate is a molecule that contains one atom of calcium, one atom of carbon, and three atoms of oxygen.
What kind of compound is CaCO3?
Calcium carbonate is a chemical compound with the formula CaCO3 formed by three main elements: carbon, oxygen, and calcium.
How do you get CaCO3?
CaCO3 is obtained by using carbon dioxide and slaked lime as raw materials. When carbon dioxide is passed through slaked lime, calcite is obtained. Another method to obtain calcite is by adding sodium carbonate to calcium chloride.
Oxidation states of carbon | Resonance and acid-base chemistry | Organic chemistry | Khan Academy
Images related to the topicOxidation states of carbon | Resonance and acid-base chemistry | Organic chemistry | Khan Academy
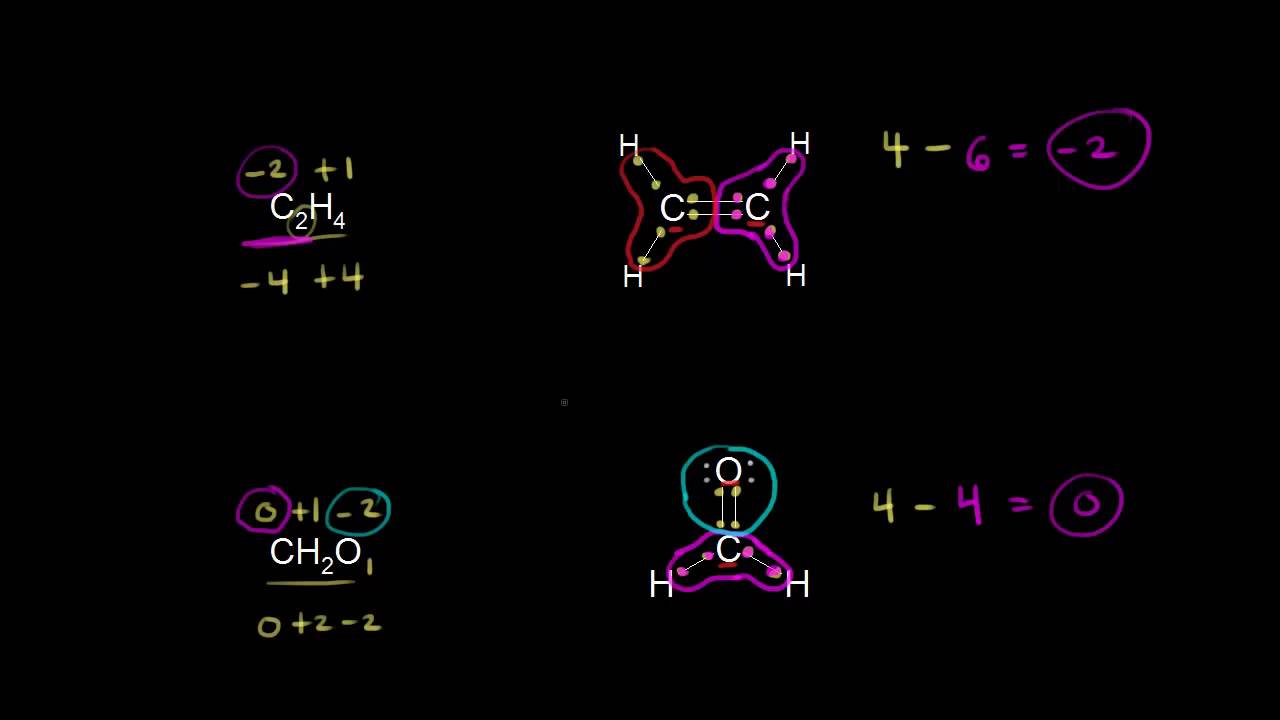
What is the oxidation state of C2 o4?
C2O4 is a polyatomic ion with a -2 charge. Within this molecule, the oxygen atom has an oxidation state of-2 as oxygen also has -2 charges on it.
What is the oxidation state of C2 h4 o2?
Since there are 4 atoms of oxygen the overall charge of the oxygen is 4 (-2) =-8. The oxidation states of c is +3 & o is -2.
Related searches to what is the oxidation state of an individual carbon atom in caco3?
- what is the oxidation state of an individual nitrogen atom in nh4oh
- oxidation number of ca
- what is the oxidation state of an individual nitrogen atom in hno3hno3 ?
- oxidation number of cao
- what is the oxidation state of an individual nitrogen atom in kno3
- what is the oxidation state of an individual nitrogen atom in hno3hno3
- what is the oxidation state of an individual nitrogen atom in nh2oh ?
- find the oxidation number of caco3
- what is the oxidation state of an individual nitrogen atom in nh2oh
- oxidation number of carbon
- what is the oxidation state of an individual nitrogen atom in kno3 ?
- what is the oxidation state of an individual nitrogen atom in nh4oh ?
Information related to the topic what is the oxidation state of an individual carbon atom in caco3?
Here are the search results of the thread what is the oxidation state of an individual carbon atom in caco3? from Bing. You can read more if you want.
You have just come across an article on the topic what is the oxidation state of an individual carbon atom in caco3?. If you found this article useful, please share it. Thank you very much.