Are you looking for an answer to the topic “what is the empirical formula for c4h10“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
The molecular formula of butane is C 4H 10. This is the actual number of atoms of each element in a molecule of butane. This formula does not show the simplest whole number ratio because each number can be divided by 2. This gives the empirical formula of butane – C 2H 5.Butane () or n-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature.
- In any empirical formula problem you must first find the mass % of the elements in the compound. …
- Then change the % to grams. …
- Next, divide all the masses by their respective molar masses. …
- Pick the smallest answer of moles and divide all figures by that.

How do you calculate the empirical formula?
- In any empirical formula problem you must first find the mass % of the elements in the compound. …
- Then change the % to grams. …
- Next, divide all the masses by their respective molar masses. …
- Pick the smallest answer of moles and divide all figures by that.
What type of formula is C4H10?
Butane () or n-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature.
How to Write the Empirical Formula for Butane (C4H10)
Images related to the topicHow to Write the Empirical Formula for Butane (C4H10)

Is ch4 an empirical formula?
Is C2H4 an empirical formula?
While C2H4 is its molecular formula and represents its true molecular structure, it has an empirical formula of CH2. The simplest ratio of carbon to hydrogen in ethene is 1:2.
What is the value of c4h10?
…
3.1Computed Properties.
| Property Name | Property Value | Reference |
|---|---|---|
| Monoisotopic Mass | 58.078250319 | Computed by PubChem 2.1 (PubChem release 2021.05.07) |
What is molar mass of c4h10?
Is c4h10 alkane alkene or alkyne?
Since C4H10 follows the general formula CnH2n-2 therefore it is an alkane.
See some more details on the topic what is the empirical formula for c4h10 here:
What is the empirical formula for C4H10? | Study.com
The empirical formula for C4 H10 is C2 H5 . We can simplify the molecular formula C4 H10 , which is the formula for butane, by dividing the formula…
How can I calculate the empirical formula of butane? | Socratic
It depends on the information you have. The empirical formula tells us the simplest whole-number ratio of the different types of atoms in a …
What is the empirical formula for C4H10 – Atoms and Molecules
the molecular formula of butane is C4H10 while its empirical formula is C2H5. since the empirical formulas are the simplest ratios.
What is the difference between the empirical formula and …
For example, the alkane butane has a molecular formula of C4H10. One molecule of Butane contains 4 Carbon atoms and 10 Hydrogen atoms.
What is the molecular and empirical formula of CH4?
What is empirical formula with example?
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2.
Empirical Formula Molecular Formula Determination From Percent Composition
Images related to the topicEmpirical Formula Molecular Formula Determination From Percent Composition
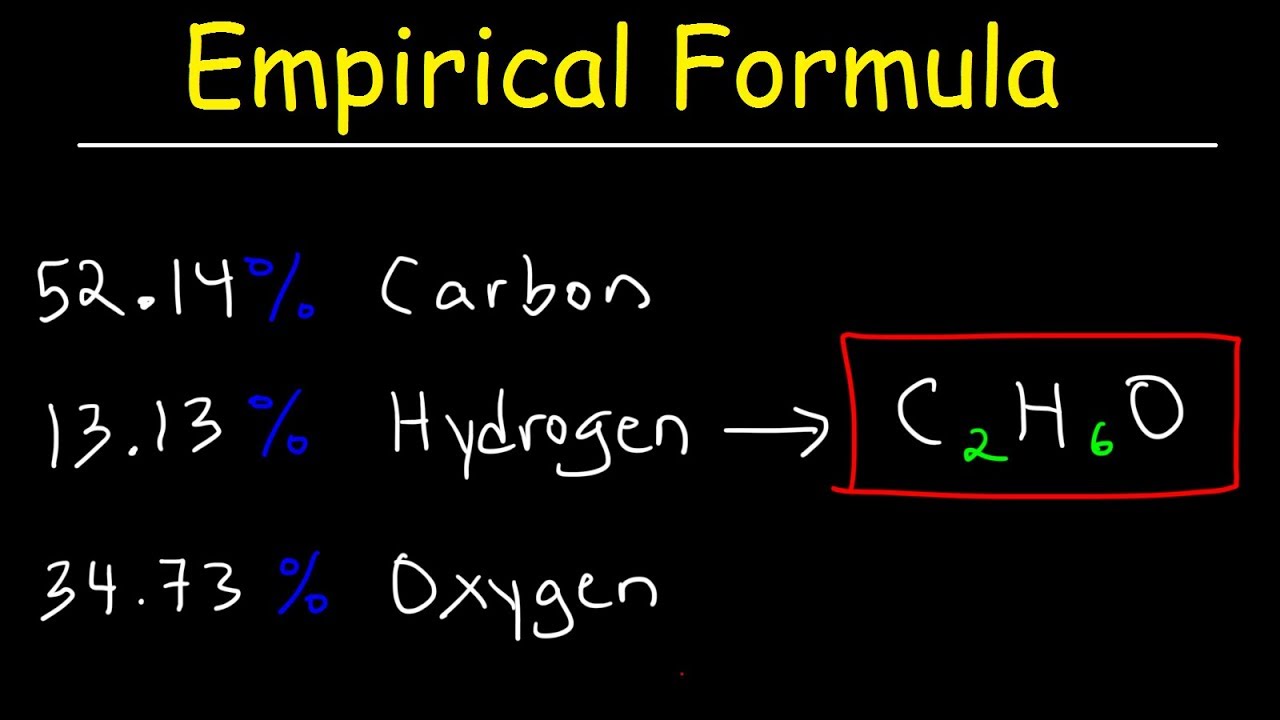
What is the empirical formula of CH3?
Since there are 2 H atoms per H2O molecule, this is 1/2 the amount of H in the original compound. (12.59 g H2O)(1 mol/18.02 g) = 0.6987 mol H2O therefore twice as much H (1.397 mol) in the original compound. Therefore, CH3 is the empirical formula. CH3 molar mass = 1C + 3H = (12.01) + 3(1.01) = 15.04 g/mol.
What is the empirical formula of C2H2?
What is the empirical formula? C2H2 is divisible by “n ratio factor” of two; thus C1H1 is the empirical formula.
Which of the following is an empirical formula?
C3H8 is an example of an empirical formula. It represents the relative number of atoms in the simplest ratio. Others are examples of molecular formula. They represents the actual number of constituent atoms in a molecule.
Is C4H8 an empirical formula?
The empirical formula is a formula that gives the simplest whole number ratio of atoms in a compound. For example, C2H4, C3H6, and C4H8 all contain twice as many H atoms than C atoms. Therefore, the empirical formula of all these molecules is CH2.
What is the structural formula of?
Structural formulas identify the location of chemical bonds between the atoms of a molecule. A structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds—one, two, or three lines standing for single, double, or triple bonds, respectively. For example,…
How do you calculate empirical and molecular formulas?
STEP 1: Calculate the molar mass of the empirical formula. STEP 2: Divide the given molecular molar mass by the molar mass calculated for the empirical formula. STEP 3: Multiply each subscript by the whole number that resulted from step 2. This is now the molecular formula.
What is empirical formula with example?
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2.
Empirical Formula and Molecular Formula Introduction
Images related to the topicEmpirical Formula and Molecular Formula Introduction
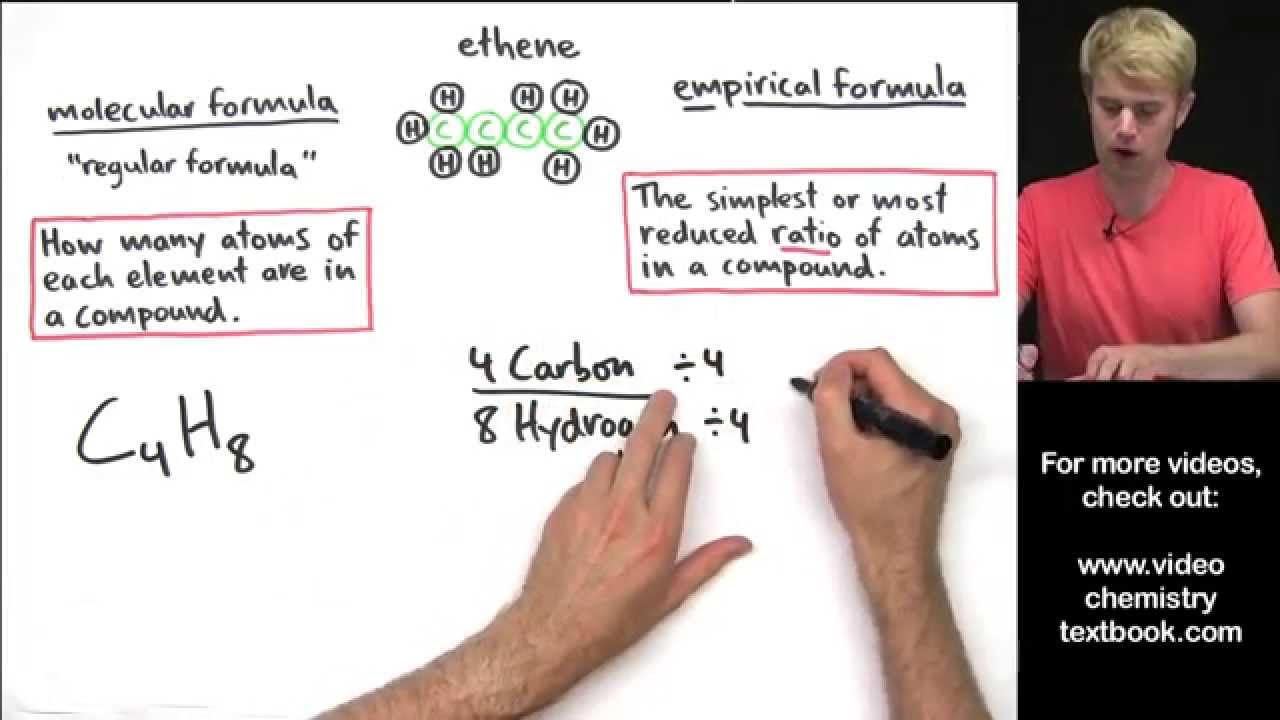
How do you find the empirical formula from a table?
Convert the mass of each element to moles using the molar mass from the periodic table. Divide each mole value by the smallest number of moles calculated. Round to the nearest whole number. This is the mole ratio of the elements and is represented by subscripts in the empirical formula.
What is empirical formula mean?
Definition of empirical formula
: a chemical formula showing the simplest ratio of elements in a compound rather than the total number of atoms in the molecule CH2O is the empirical formula for glucose.
Related searches to what is the empirical formula for c4h10
- what does the empirical formula tell us about butane
- c3h8 empirical formula
- isobutane empirical formula
- c6h12o6 empirical formula
- does c4h10 and c10h4 have the same empirical formula
- how to find empirical formula
- all of the following are empirical formula except
- what is the empirical formula for c4h10o
- what is the empirical formula for c4h10o2 quizlet
- empirical formula of ethane
- what is the empirical formula for c2h4o2
- what is the empirical formula for c4h10o2
- what is the empirical formula for c4h10o2 group of answer choices
Information related to the topic what is the empirical formula for c4h10
Here are the search results of the thread what is the empirical formula for c4h10 from Bing. You can read more if you want.
You have just come across an article on the topic what is the empirical formula for c4h10. If you found this article useful, please share it. Thank you very much.