Are you looking for an answer to the topic “pka to ka“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
To create a more manageable number, chemists define the pKa value as the negative logarithm of the Ka value: pKa = -log Ka. If you already know the pKa value for an acid and you need the Ka value, you find it by taking the antilog.For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.More precisely – pKa is the negative log base ten of the Ka value (acid dissociation constant). It measures the strength of an acid — how tightly a proton is held by a Bronsted acid. The lower the value of pKa, the stronger the acid and the greater its ability to donate its protons.

Is Ka equal to pKa?
For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.
What is Ka and pKa value?
More precisely – pKa is the negative log base ten of the Ka value (acid dissociation constant). It measures the strength of an acid — how tightly a proton is held by a Bronsted acid. The lower the value of pKa, the stronger the acid and the greater its ability to donate its protons.
pKa, Ka, and Acid Strength
Images related to the topicpKa, Ka, and Acid Strength

Is pKa inversely related to Ka?
Ka and Pka are two reciprocal terms if one is higher, then the other will be lower itself. Ka is directly related to acidity, and Pka is inversely related to acidity thus helps in basicity.
How do I calculate Ka?
To find out the Ka of the solution, firstly, we will determine the pKa of the solution. At the equivalence point, the pH of the solution is equivalent to the pKa of the solution. Thus using Ka = – log pKa equation, we can quickly determine the value of Ka using a titration curve.
How do you convert pKa to pKb?
Steps to Convert between pKa and pKb
Step 1: Write the equation that relates pKa p K a and pKb p K b . It should look like the following: pKa+pKb=14 p K a + p K b = 14 . Step 2: Insert the given pK value provided in the question.
How do you convert pH to Ka?
As noted above, [H3O+] = 10–pH. Since x = [H3O+] and you know the pH of the solution, you can write x = 10–2.4. It is now possible to find a numerical value for Ka. Ka = (10–2.4)2 /(0.9 – 10–2.4) = 1.8 x 10–5.
What is Ka value?
What is the Ka value? The acid dissociation constant (Ka) is used to distinguish strong acids from weak acids. Strong acids have exceptionally high Ka values. The Ka value is found by looking at the equilibrium constant for the dissociation of the acid. The higher the Ka, the more the acid dissociates.
See some more details on the topic pka to ka here:
How to Convert pKa to Ka – Sciencing
To create a more manageable number, chemists define the pKa value as the negative logarithm of the Ka value: pKa = -log Ka.
How do you convert pKa to Ka? | Socratic
⇒Ka=10−(pKa). Explanation: pKa=−log10Ka ⇒−pKa=log10Ka ⇒10−(pKa)=Ka ⇒Ka=10−(pKa).
How to convert PKA to KA – eHow UK
Write down the relationship between pKa and Ka. According to the Chem Tutor website, this is expressed by the equation pKa = -log(Ka).
A Simple Method That Shows You How to Convert pKa to Ka …
The term ‘pKa’ is more commonly used in chemical calculations as compared to ‘Ka’. Despite this, there is a lot of confusion between the two concepts.
When pKa value is small Ka value is?
pKa and Ka
A small Ka value means the reaction favors the reactants rather than the products. Most weak acids have Ka values between 10–2 to 10–14. pKa gives the same information, but in a different way. The smaller the pKa value, the stronger the acid.
Ka and pKa Derivation
Images related to the topicKa and pKa Derivation
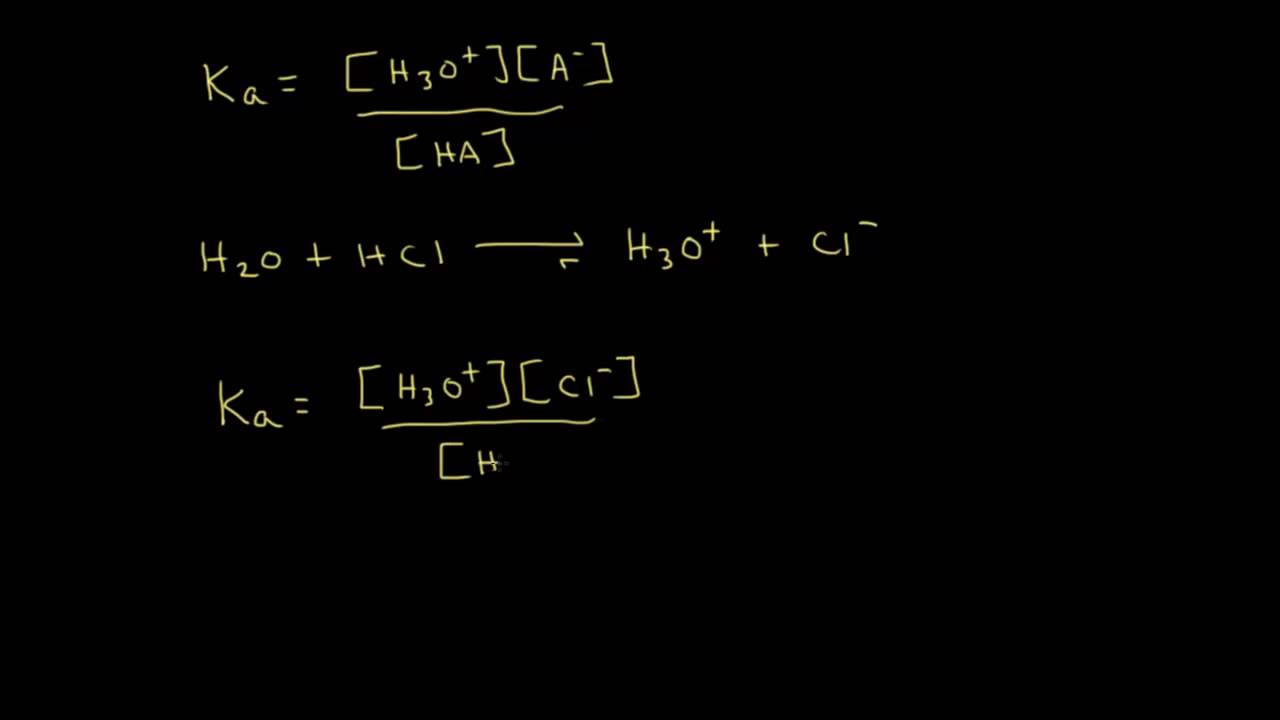
Is pKa the same as pH?
The pH is a measure of the concentration of hydrogen ions in an aqueous solution. pKa (acid dissociation constant) and pH are related, but pKa is more specific in that it helps you predict what a molecule will do at a specific pH.
How do you find Ka and KB values?
Solve the equation for Kb by dividing the Kw by the Ka. You then obtain the equation Kb = Kw / Ka. Put the values from the problem into the equation. For example, for the chloride ion, Kb = 1.0 x 10^-14 / 1.0 x 10^6.
How do you find the Ka value of an unknown acid?
In today’s experiment you will first determine Ka of an unknown acid by measuring the pH of the pure acid (no salt present). Next you will titrate the acid to find what volume of base is needed to neutralize it completely. each solution, you will calculate Ka.
What is the relationship between pKa and pKb?
Summary – pKa vs pKb
pKb is given for dissociation of bases. The difference between pKa and pKb is that pKa is the negative logarithm of Ka whereas pKb is the negative logarithm of Kb.
How do you convert pH to pKb?
- Find the [OH-] from the pKb value (in the same way as for weak acid type calculations)
- From the [OH-] find the pOH.
- Find the pH from: pOH + pH = 14.
Can you use pKb in Henderson Hasselbalch equation?
Calculate the pH of an alkaline (or basic) buffer solution. You can rewrite the Henderson-Hasselbalch equation for bases: pOH = pKb + log ([B+]/[BOH]), where “pKb” is the base’s dissociation constant, “[B+]” stands for the concentration of a base’s conjugate acid and “[BOH]” is the concentration of the base.
How are Ka and pKa values related to strength of an acid?
Relationship between Ka, pKa and acid strength: The smaller the value of Ka, the larger the value of pKa, the weaker the acid. If the pH of a solution of a weak acid and the pKa are known, the ratio of the concentration of the conjugate base to the concentration of the acid may be calculated.
How to convert between Ka and pKa (or Kb and pKb)
Images related to the topicHow to convert between Ka and pKa (or Kb and pKb)

Does high Ka mean low pH?
Explanation: The Ka is the acid dissociation constant, and thus it is what determines how strong the acid is. Stronger acids dissociate to a greater extent and produce lower pH values.
Is pKa the same as pH?
The pH is a measure of the concentration of hydrogen ions in an aqueous solution. pKa (acid dissociation constant) and pH are related, but pKa is more specific in that it helps you predict what a molecule will do at a specific pH.
Related searches to pka to ka
- pka to ka converter
- pka=-logka which equation
- pka to ka without calculator
- pka to ka mcat
- kam karne ka formula
- pka to ka
- how to get ka
- pka to ka chemistry
- pKa to Ka
- how to find ka from pka without calculator
- pka to ka conversion calculator
- pka to ka sig figs
- pka and ka relationship
- the equation pka log ka is known as
- how is pka related to ka
- pka convert to ka
- ka 10 pka
- pka to ka relationship
- how to convert pka to ka on calculator
- pka is negative log of ka
- how to convert pka to ka
- ka = 10-pka
- pka to ka online calculator
- pka logka which equation
- the equation pka–log ka is known as:
- how to go from pka to kb
Information related to the topic pka to ka
Here are the search results of the thread pka to ka from Bing. You can read more if you want.
You have just come across an article on the topic pka to ka. If you found this article useful, please share it. Thank you very much.
