Are you looking for an answer to the topic “p4 polar or nonpolar“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
In P4, all of the atoms bonding are the same. There will be no polarity between in the bonds between the atoms, and thus no polarity in the compound as a whole. Therefore, tetraphosphorus is nonpolar since each phosphorus atom’s electron pull in opposing directions is the same and thus cancel each other out.The Phosphorus P4 molecule contains a total of 3 bond(s) There are 3 non-H bond(s), 3 multiple bond(s), 1 double bond(s) and 2 triple bond(s). The 2D chemical structure image of Phosphorus P4 is also called skeletal formula, which is the standard notation for organic molecules.The Sulfur tetrafluoride is a polar molecule because Fluorine is more electronegative than Sulfur. With this, the distribution of the charge is not equal, making the SF4 polar molecules.

What type of bond is P4?
The Phosphorus P4 molecule contains a total of 3 bond(s) There are 3 non-H bond(s), 3 multiple bond(s), 1 double bond(s) and 2 triple bond(s). The 2D chemical structure image of Phosphorus P4 is also called skeletal formula, which is the standard notation for organic molecules.
Is s4 polar or nonpolar?
The Sulfur tetrafluoride is a polar molecule because Fluorine is more electronegative than Sulfur. With this, the distribution of the charge is not equal, making the SF4 polar molecules.
Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar
Images related to the topicPolar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar

Is phosphorus a polar?
The oxygen-phosphorous bonds are covalent but polar, the molecule its-self is charged, but lacks a direction across the molecule of the charge making phosphate non-polar. The molecule its-self is free to bond covalent or ionicly depending on what it reacts with. A.K.
Is P4 ionic or covalent?
411A: M2, U4, P4 : The Polar Covalent Bond.
What is P4 in chemistry?
Phosphorus P4 | P4 – PubChem.
What is the structure of P4?
White phosphorus, yellow phosphorus or simply tetraphosphorus (P4) exists as molecules made up of four atoms in a tetrahedral structure. The tetrahedral arrangement results in ring strain and instability. The molecule is described as consisting of six single P–P bonds.
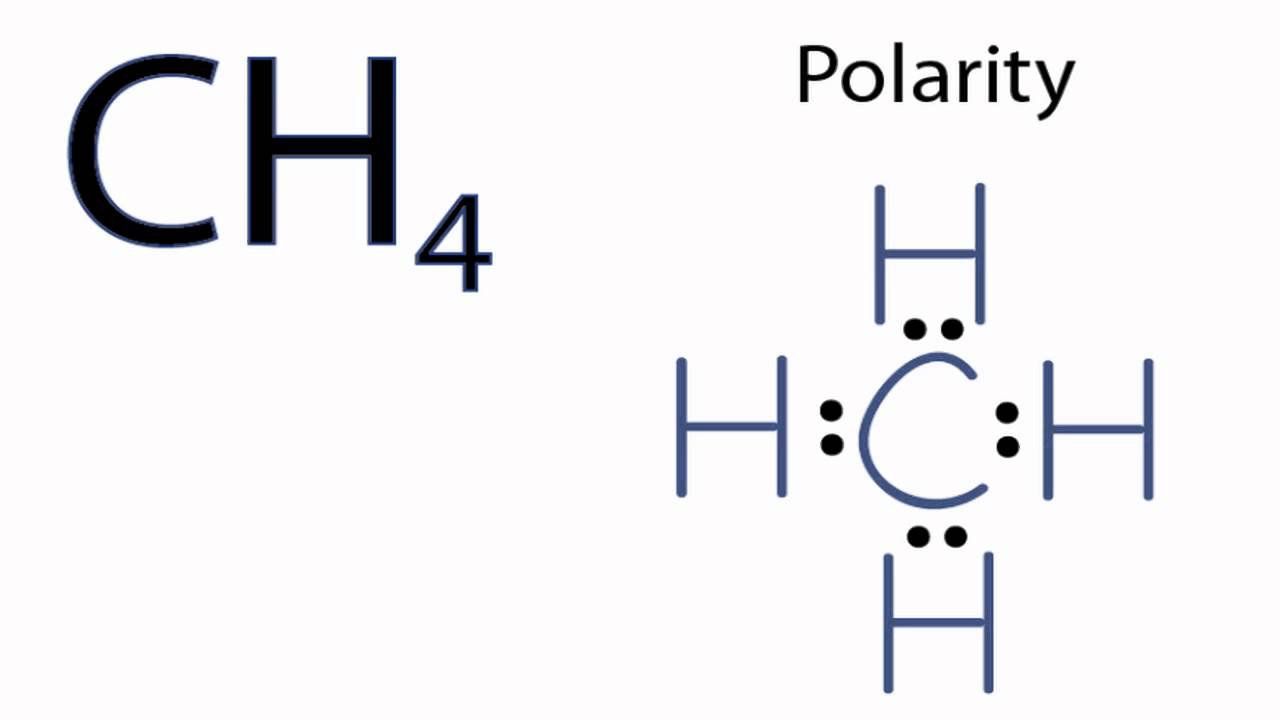
Is CH4 polar covalent?
Methane (CH4) is a non-polar hydrocarbon compound composed out of a single carbon atom and 4 hydrogen atoms. Methane is non-polar as the difference in electronegativities between carbon and hydrogen is not great enough to form a polarized chemical bond.
See some more details on the topic p4 polar or nonpolar here:
Is P4 polar? – Quora
p4 is non polar covalent tetrahedron. This is because all the bonded atoms are of same electronegativity.
Why P4 not polar? – CHEMISTRY COMMUNITY – Laurence …
Answer: In P4, all of the atoms bonding are the same. There will be no polarity between in the bonds between the atoms, and thus no polarity …
Is P4 polar or nonpolar ? – Bengis Life
Answer = P4 ( Phosphorus tetramer ) is Nonpolar … What is polar and non-polar? Polar … Polar molecules must contain polar bonds due to a difference in …
What kind of bond is P4? – Best Acting Colleges In New York
a) P4: is a molecular non-polar (pure) covalent molecule. … It has bonds between all molecules in three dimensions. Is PCl3 …
Why is SF4 a polar molecule?
The geometry of Sf4 will be asymmetric electron region distribution around the central atom (S). Therefore this molecule is polar.
Is po4 polar or nonpolar?
Answer and Explanation: The polyatomic ion phosphate is non-polar due to its symmetrical, tetrahedral shape and even distribution of charges.
Why is po4 polar?
Phosphate R-PO
A phosphate group is an phosphorus atom covalently bound to 4 oxygen atoms and contains one P=O. bond and three P-O− bonds. The oxygen atoms are more electronegative than the phosphorous atom resulting in polar covalent bonds.
Why is a phosphate polar?
A single phospholipid molecule has a phosphate group on one end, called the “head,” and two side-by-side chains of fatty acids that make up the lipid “tails. ” The phosphate group is negatively charged, making the head polar and hydrophilic, or “water loving.” The phosphate heads are thus attracted to the water …
Is P4 a molecular solid or covalent?
P4 molecule is a molecular solid which is a polymer.it is a molecular solid as the constituent particles in the solid are P4 molecules.
Is P4 a covalent molecular solid?
Answer. Answer: P4: is a molecular non-polar (pure) covalent molecule.
Polar Non-Polar Molecules: Crash Course Chemistry #23
Images related to the topicPolar Non-Polar Molecules: Crash Course Chemistry #23

Is P4 a molecular compound?
P4 is a substance that is made up of four atoms of the same element, so it is a molecular element.
Is P4 same as 4P?
What is the difference between P4 and 4P ? P4 is a phosphorus molecule. … Since, the number 4 is before the P atom it is representing that there are 4 atoms of phosphorus element. P4 is representing the Phosphorus molecule and 4P is representing the P atom.
Is P4 a diatomic molecule?
Nitrogen exists as diatomic molecule and phosphorus as P4.
What does P4 look like?
This looks much like “yellow phosphorus” with a thin coating of phosphorus oxides, P4O6 and P4O10. The yellow appearance is explained in several different ways in the literature: Partington3 stated: “White phosphorus is commonly a white translucent soft waxy solid … On exposure to light it becomes yellow.”
Why is phosphorus called P4?
Answer: Phosphorus can form a P4 white phosphorus tetrahedron because it can form three bonds. The most stable allotrope of phosphorus, red phosphorus, is a cross-linked, polymeric chain of atoms. P has a high atomic size and a low tendency for forming triple bonds.
Is P4 tetrahedral?
In P4. molecule phosphorus atoms are tetrahedrally arranged. The bond angle ∠P−P−P in the molecule is. Hint: White phosphorus, yellow phosphorus or simply tetraphosphorus exists as molecules made up of four atoms in a tetrahedral structure.
Is phosphorus a P4 or P?
phosphorus (P), nonmetallic chemical element of the nitrogen family (Group 15 [Va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark.
Is CH4 polar or nonpolar electronegativity?
CH4 is a nonpolar molecule because of its symmetric tetrahedral geometrical form and four identical C-H bonds. Carbon and hydrogen have electronegativity values of 2.55 and 2.2, respectively, resulting in almost zero partial charges.
What type of bond is CH4?
Methane, CH4, is a covalent compound with exactly 5 atoms that are linked by covalent bonds. We draw this covalent bonding as a Lewis structure (see diagram). The lines, or sticks, as we say, represent the covalent bonds. There are four bonds from a central carbon (C) linking or bonding it to four hydrogen atoms (H).
Is CH4 ionic or covalent?
Methane, CH4, is a covalent compound with exactly 5 atoms that are linked by covalent bonds. We draw this covalent bonding as a Lewis structure (see diagram). The lines, or sticks, as we say, represent the covalent bonds. There are four bonds from a central carbon (C) linking or bonding it to four hydrogen atoms (H).
What is the name of P4?
Species with the same structure: P4.
Is CH4 (Methane) Polar or Nonpolar?
Images related to the topicIs CH4 (Methane) Polar or Nonpolar?
Is boron trifluoride polar or nonpolar?
Boron trifluoride (BF3) is a nonpolar molecule, whereas ammonia (NH3) is a polar molecule.
Is BF3 ionic or covalent?
Boron trifluoride (BF3) is a covalent compound.
Related searches to p4 polar or nonpolar
- is cl2 polar or nonpolar
- is p4 polar
- is p4 polar covalent
- is bcl3 polar or nonpolar
- is np polar or nonpolar
- is co polar or nonpolar
- n2 polar or nonpolar
- p4o6 polar or nonpolar
- p4 polar or nonpolar or ionic
- is h2s polar or nonpolar
- nh3 polar or nonpolar
- p4o7 polar or nonpolar
- co2 polar or nonpolar
- is p4 ionic polar covalent or nonpolar covalent
- p4 lewis structure polar or nonpolar
- is no2 polar or nonpolar
- p4o10 polar or nonpolar
- does n2 have polar or nonpolar bonds
Information related to the topic p4 polar or nonpolar
Here are the search results of the thread p4 polar or nonpolar from Bing. You can read more if you want.
You have just come across an article on the topic p4 polar or nonpolar. If you found this article useful, please share it. Thank you very much.