Are you looking for an answer to the topic “ksp vs keq“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Ksp stands for solubility product constant while Keq stands for equilibrium constant. The key difference between Ksp and Keq is that the term Ksp describes the solubility of a substance, whereas the term Keq describes the equilibrium state of a particular reaction.What is Ksp? Ksp (Solubility product constant) is the equilibrium between a solid and its respective ions in a solution. The value of the constant identifies the degree of which the compound can dissociate in water. For example the higher the Ksp the more soluble the compound is.The solubility product constant (Ksp) describes the equilibrium between a solid and its constituent ions in a solution. The value of the constant identifies the degree to which the compound can dissociate in water. The higher the Ksp, the more soluble the compound is.
- Ksp = [0.0159][0.0318]2 = 1.61 x 10–5 Top.
- 1.1 x 10–12 = [2x]2[x] x = 6.50 x 10–5 M. …
- 1.1 x 10–10 = [x][0.020 + x] = [x][0.020] x = 5.5 x 10–9 M. …
- Q = (0.0000938 M Pb2+)(0.00050 M CrO42–) = 4.69 x 10–8
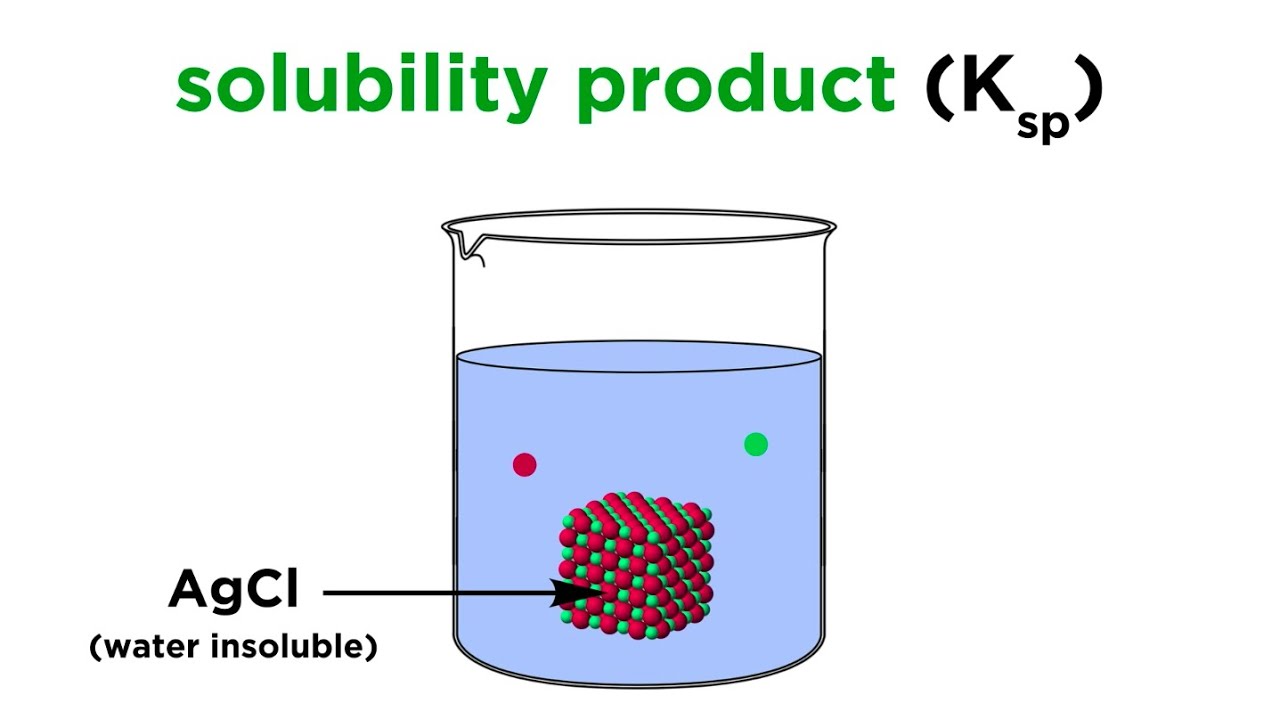
What is Ksp equal to?
What is Ksp? Ksp (Solubility product constant) is the equilibrium between a solid and its respective ions in a solution. The value of the constant identifies the degree of which the compound can dissociate in water. For example the higher the Ksp the more soluble the compound is.
What does Ksp tell you in terms of equilibrium?
The solubility product constant (Ksp) describes the equilibrium between a solid and its constituent ions in a solution. The value of the constant identifies the degree to which the compound can dissociate in water. The higher the Ksp, the more soluble the compound is.
Solubility Product Constant (Ksp)
Images related to the topicSolubility Product Constant (Ksp)

How do you find equilibrium constant from Ksp?
- Ksp = [0.0159][0.0318]2 = 1.61 x 10–5 Top.
- 1.1 x 10–12 = [2x]2[x] x = 6.50 x 10–5 M. …
- 1.1 x 10–10 = [x][0.020 + x] = [x][0.020] x = 5.5 x 10–9 M. …
- Q = (0.0000938 M Pb2+)(0.00050 M CrO42–) = 4.69 x 10–8
How does Ksp compare to solubility?
The relative MOLAR solubility of salts (saturated solution) can be determined by comparing Ksp values. The greater the Ksp the more ions are in solution, hence the greater the molar solubility.
What is the relationship between the solubility product constant Ksp and temperature?
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
What is the relationship between Ksp and molar solubility?
A substance’s solubility product (Ksp) is the ratio of concentrations at equilibrium. Molar solubility, which is directly related to the solubility product, is the number of moles of the solute that can be dissolved per liter of solution before the solution becomes saturated.
What does Ksp stand for?
| Acronym | Definition |
|---|---|
| KSP | Solubility Product Constant |
| KSP | Key Stone Project (Japan) |
| KSP | Kulang Sa Pansin (song) |
| KSP | Karnataka State Police (India) |
See some more details on the topic ksp vs keq here:
Ksp vs Keq | Physics Forums
Ksp and Keq are the same thing – equilibrium constant of some process. However, Ksp describes saturated solution, so it doesn’t hold when there …
The Keq family
Ksp comes from Keq, only this time our reactants are solid so they get left out. That is because for a solid concentration is meaningless.
Solubility Product Constant, Ksp – Chemistry LibreTexts
The solubility product constant, Ksp, is the equilibrium constant for a … effect on the Ksp value compared to the common ion effect, …
Keq vs. Kc vs. Kp – CHEMISTRY COMMUNITY – Laurence …
Keq is the general equilibrium constant as you mentioned. Kc refers to when you are using the equilibrium concentration values to find the …
How do you determine if a precipitate will form from Ksp?
If the value of the ion product is greater than the value of the Ksp, then a precipitate will form. The formation of the precipitate lowers the concentration of each of the ions until the ion product is exactly equal to the Ksp, at which point precipitation ceases.
What does the solubility product constant Ksp represent?
The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp value it has.
How do you find Ksp expression?
Ksp=[M+][X−][MX(s)] , but MX(s) as A SOLID cannot express a concentration, and thus the expression simplifies to… Usually standard conditions are specified, because a hot solution can generally hold more solute than a cold one. have been measured for a host of insoluble, and semi-soluble ionic salts.
Is K and KC the same?
The key difference between Kc and Kp is that Kc is the equilibrium constant which is given by the terms of concentration whereas Kp is the equilibrium constant which is given by the terms of pressure.
Ksp – Molar Solubility, Ice Tables, Common Ion Effect
Images related to the topicKsp – Molar Solubility, Ice Tables, Common Ion Effect
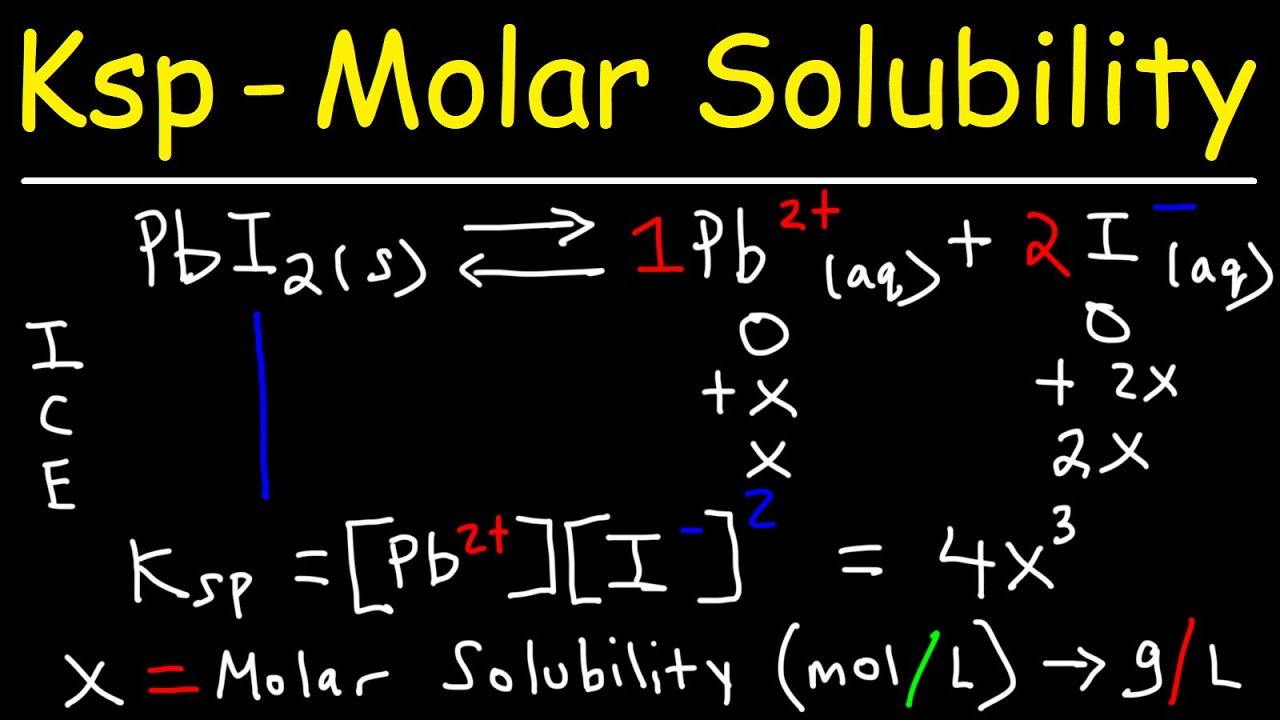
What does Keq depend on?
As detailed in the above section, the position of equilibrium for a given reaction does not depend on the starting concentrations and so the value of the equilibrium constant is truly constant. It does, however, depend on the temperature of the reaction.
What happens when QSP is greater than Ksp?
When QSP is greater than KSP, the solution is oversaturated. So it’s exceeded the limit of what can dissolve, and therefore you can imagine some lead two plus ions combining with some sulfate ions to form a precipitate. Therefore, when QSP is greater than KSP, a precipitate will form.
What does Le Chatelier’s principle say?
– [Instructor] Le Chatelier’s principle says, if a stress is applied to a reaction mixture at equilibrium, the net reaction goes in the direction that relieves the stress. Change in the concentration of a reactant or product is one way to place a stress on a reaction at equilibrium.
What is the relationship between Ksp and temperature?
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
What does low Ksp mean?
The larger the negative exponent the less soluble the compound is in solution. The larger the real value of the Ksp the more soluble the compound is in solution 2.5 x 10−3 > 2.5 x 10−6. Aug 18, 2016. Ksp refers to the solubility product . It is another equilibrium expression.
What affects Ksp?
For a given chemical species and solvent system, the main factor which affects the value of Ksp is the temperature. Most often, an increase in the temperature causes an increase in the solubility and value.
How do you calculate solubility product constant from molar solubility?
…
Calculating the Ksp from the Molar Solubility.
| Molar Solubility | Ksp | |
|---|---|---|
| Example #8: Hg2I2 | 2.37 x 10¯10 M | 5.33 x 10¯29 |
What is the relationship between molar solubility and solubility product for salt given below pbi2?
Solution. The CORRECT relationship between molar solubility (S) and solubility product (Ksp) for salt, Cr(OH)3 is Ksp = 27S4.
Is solubility and molarity the same?
Molarity is a measure of concentration, i.e. Number of molesVolume of solution . Its units are thus mol⋅L−1 . Solubility is typically defined as the ability of a substance (gas, liquid, or solid) to dissolve in a solvent (typically a liquid).
How is Ksp related to K?
The solubility product constant, Ksp, is the equilibrium constant for a solid substance dissolving in an aqueous solution. It represents the level at which a solute dissolves in solution. The more soluble a substance is, the higher the Ksp value it has. Note that the reactant, aA, is not included in the Ksp equation.
What is Ksp? (Solubility Product Constant)
Images related to the topicWhat is Ksp? (Solubility Product Constant)

Is Ksp the same as molar solubility?
A substance’s solubility product (Ksp) is the ratio of concentrations at equilibrium. Molar solubility, which is directly related to the solubility product, is the number of moles of the solute that can be dissolved per liter of solution before the solution becomes saturated.
How do you find Ksp expression?
Ksp=[M+][X−][MX(s)] , but MX(s) as A SOLID cannot express a concentration, and thus the expression simplifies to… Usually standard conditions are specified, because a hot solution can generally hold more solute than a cold one. have been measured for a host of insoluble, and semi-soluble ionic salts.
Related searches to ksp vs keq
- ka vs ksp
- molar solubility
- keq from kb
- how to calculate keq
- what is the difference between ksp and keq
- is ksp the same as keq
- how to find equilibrium constant from ksp
- how to find keq from ksp and kf
- ksp vs keq mcat
- how to find ksp
- ka vs keq
Information related to the topic ksp vs keq
Here are the search results of the thread ksp vs keq from Bing. You can read more if you want.
You have just come across an article on the topic ksp vs keq. If you found this article useful, please share it. Thank you very much.