Are you looking for an answer to the topic “ka of water“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
Since [H2O] in pure water is 55.5 M, Ka = 1.8 x 10E-16, or pKa = 15.7 .Theapproximate values of both Ka andKb for water are 1.0 X 10-14 at 25 °C. that is different from that for species treated as solutes.The autoionization of liquid water produces OH− and H3O+ ions. The equilibrium constant for this reaction is called the ion-product constant of liquid water (Kw) and is defined as Kw=[H3O+][OH−]. At 25 °C, Kw is 1.01×10−14; hence pH+pOH=pKw=14.00.

What is the Ka and KB of water?
Theapproximate values of both Ka andKb for water are 1.0 X 10-14 at 25 °C. that is different from that for species treated as solutes.
Is KW the Ka of water?
The autoionization of liquid water produces OH− and H3O+ ions. The equilibrium constant for this reaction is called the ion-product constant of liquid water (Kw) and is defined as Kw=[H3O+][OH−]. At 25 °C, Kw is 1.01×10−14; hence pH+pOH=pKw=14.00.
Waterslides at Suntago Waterpark – Park of Poland
Images related to the topicWaterslides at Suntago Waterpark – Park of Poland

Why is water not included in Ka?
The reason that water does not appear in Ka is that the activity of water is assumed to very nearly equal to 1. (This assumption is correct to the degree that the solution is di- lute.) In other words, K and Ka are effectively the same con- stant.
What is the value of Ka?
A small Ka value means little of the acid dissociates, so you have a weak acid. The Ka value for most weak acids ranges from 10–2 to 10–14.
How do you calculate KB of water?
Calculate the molal elevation constant, kb for water and the boiling point of 0.1 molal urea solution. It is given that latent heat of vaporization of water is 9.72kcal/mol at 373.15K. A) Kb=0.515Kkg/mol,Tb=373.20K.
What is the equilibrium constant of water?
Pure water undergoes a reversible reaction in which both H+ and OH– are generated. The equilibrium constant for this reaction, called the water dissociation constant, Kw, is 1.01 × 10–14 at 25 °C.
Is pH of water always 7?
Difference Between pH and Acidity
In the case of pure water, the concentration of hydrogen ions and hydroxide ions never changes, so water is always neutral regardless of whether its pH level changes. At room temperature (25 degrees Celsius) the pH of pure water is 7.
See some more details on the topic ka of water here:
What is the pKa of water? – Chemistry LibreTexts
K′a=Ka=2.8×10−8. or. pKa=7.55.
Water, Acids, and Bases
In pure water, at 25C, the [H3O+] and [OH-] ion concentrations are 1.0 x 10-7 M. The value of Kw at 25C is therefore 1.0 x 10-14. [1.0 x 10 …
Ka of water? – Chemical Forums
Ka of water? … Since [H2O] is a constant of 55 mol dm^-3, and [H+][OH-] is equivalent to Kw, which is 10^-14, then Ka = 1.8×10^-16 mol dm^-3 at …
Water autoionization and Kw (article) | Khan Academy
Let’s find out by examining the equilibrium constant for this reaction (also called the autoionization constant), which has the special symbol K w K_\text{w} Kw …
How do you find the pH of water with kW?
- Kw =[H+][OH-]
- And therefore,
- -log K = -log [H+] + -log [OH-]
- log K = log[H+] + log[OH-]
- pKw = pH = pOH.
- At 25 °C pKw = 14.00 (1.0 X 10–14)
- Thus pH = pOH = 14 at 25 °C.
How do you convert pH to Ka?
As noted above, [H3O+] = 10–pH. Since x = [H3O+] and you know the pH of the solution, you can write x = 10–2.4. It is now possible to find a numerical value for Ka. Ka = (10–2.4)2 /(0.9 – 10–2.4) = 1.8 x 10–5.
Is water included in KC?
Here, however, you are considering the equilibrium constant of an esterification so you need to use Kc because water is not in excess so cannot be treated as constant! Hence the expression for Kc must include the water!
Are liquids included in KC?
Pure solids and pure liquids, including solvents, are not included in the equilibrium expression. KcK, start subscript, start text, c, end text, end subscript is often written without units, depending on the textbook.
Water Parks for Kids and Splash Pads with Ryan’s Family Review!
Images related to the topicWater Parks for Kids and Splash Pads with Ryan’s Family Review!

Is aqueous included in KC?
Kc is in terms of concentration, so will include aqueous species, mixture of liquids and gases.
Does Ka change with dilution?
The ONLY factor that affect Ka is temperature, and therefore there is no influence of concentration change on the value of Ka .
Is pKa and pH the same?
The pH is a measure of the concentration of hydrogen ions in an aqueous solution. pKa (acid dissociation constant) and pH are related, but pKa is more specific in that it helps you predict what a molecule will do at a specific pH.
What is the mass of water?
What is a KB value?
Kb value refers to a standardized ASTM test that measures the relative strength of a non-aqueous cleaning fluid. The test involves measuring the solubility of a very specific type of contamination, called “kauri gum.” Kb values range from 10 (very mild) to 200 or even higher (very strong).
How do you calculate molality from KB?
Insert the ebullioscopic constant or boiling point elevation constant, Kb = 0.512 °C⋅kg/mol . Fill in the molality of the solution, m = 3 . Using the boiling point elevation equation: ΔT = i * Kb * m = 1 * 0.512 * 3 = 1.536 °C …and boiling point of the solution is: Tsolution = Tsolvent + ΔT = 100 + 1.536 = 101.536 °C.
How do you find ka?
To find out the Ka of the solution, firstly, we will determine the pKa of the solution. At the equivalence point, the pH of the solution is equivalent to the pKa of the solution. Thus using Ka = – log pKa equation, we can quickly determine the value of Ka using a titration curve.
Why is concentration of water constant?
As water is very weak electrolyte it dissociate to very less extent ie out of 550 million moles just one dissociate. Hence its concentration is almost constant.
What is the concentration of water?
The standard state for a liquid is the pure liquid, so the standard state of water is pure water, whose concentration is 55.5 M (in a liter, there are 55.5 moles of water, so its concentration is 55.5 mol/L).
What is the pH of pure water?
The measurement of alkalinity and pH is needed to determine the corrosivity of the water. The pH of pure water (H20) is 7 at 25 °C, but when exposed to the carbon dioxide in the atmosphere this equilibrium results in a pH of approximately 5.2 because CO2 in the air dissolves in the water and forms carbonic acid.
pH of Weak Acids and Bases – Percent Ionization – Ka Kb
Images related to the topicpH of Weak Acids and Bases – Percent Ionization – Ka Kb
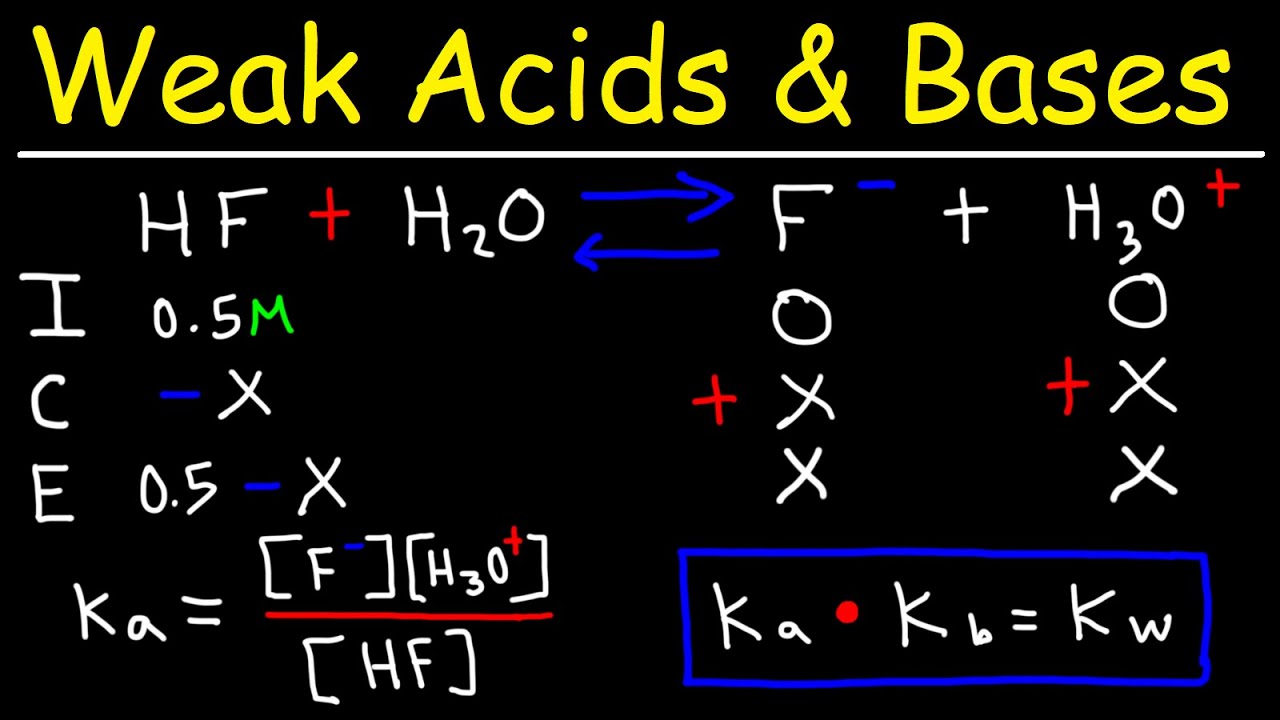
What is a pH of water?
Pure water has a pH of 7 and is considered “neutral” because it has neither acidic nor basic qualities.
Why is water neutral pH?
A solution is acidic if there is an excess of hydrogen ions over hydroxide ions (i.e., pH < pOH). In the case of pure water, there are always the same concentration of hydrogen ions and hydroxide ions and hence, the water is still neutral (pH = pOH) – even if its pH changes.
Related searches to ka of water
- kannada meaning of water
- pka h2o
- kw of water
- kaminari is afraid of water fanfiction
- write the ka expression for each of the following in water
- kansas department of water
- ka and kb of water
- kb of oh
- kansas division of water resources
- ka of ch3oh
- water has a ka value of
- karachi supply of water
- kb of oh-
- pkw water
- water kettle
- ford ka water leak back of engine
- kanye bottle of water tweet
- kauai department of water
- ka of distilled water
- pka of water
- how to calculate ka of water
- kanji of water
- kanye bottle of water
- bring a glass of water ka passive voice
- ka value of water
- ka of water at 25 c
Information related to the topic ka of water
Here are the search results of the thread ka of water from Bing. You can read more if you want.
You have just come across an article on the topic ka of water. If you found this article useful, please share it. Thank you very much.