Is PCl5 an octet or duet rule?
In PCl5, each chlorine atom happily obeys the octet rule, sporting a full set of eight electrons (four pairs). But phosphorus is a bit of a rule-breaker. It ends up with ten electrons (five pairs) in its outer shell.
Now, why does this happen? It all boils down to phosphorus’s ability to expand its valence shell. Phosphorus, unlike many other elements, can accommodate more than eight electrons in its outer shell. This is because it has empty d-orbitals, which can hold extra electrons.
Imagine a spacious room with extra furniture – that’s phosphorus’s expanded valence shell. This allows it to form five bonds with chlorine, exceeding the usual octet limit.
To understand this better, let’s visualize the bonding in PCl5. Phosphorus has five valence electrons, and each chlorine atom has seven. Phosphorus shares one electron with each chlorine atom, forming five single covalent bonds. Each chlorine atom receives one electron from phosphorus, completing its octet. However, phosphorus ends up with ten electrons in its outer shell – two from its original valence electrons plus one from each of the five chlorine atoms.
So, while phosphorus breaks the octet rule, it’s not breaking any chemical laws. It’s simply taking advantage of its unique ability to expand its valence shell, allowing it to form more bonds and create a stable molecule.
Is PCl5 a super octet?
PCl5: Phosphorus, the central atom in this molecule, forms bonds with five chlorine atoms. This means it has 10 bonding electrons surrounding it. The classic octet rule states that atoms strive to have eight electrons in their outermost shell for stability. However, phosphorus can expand its octet because it has available d-orbitals which participate in bonding. These d-orbitals allow phosphorus to accommodate more than eight electrons, making it a super octet molecule.
Think of it this way: The octet rule is like a basic apartment with only four rooms. But phosphorus, being a bit more ambitious, has access to a penthouse with extra rooms (d-orbitals). These extra rooms allow it to fit more guests (electrons) without overcrowding.
Here’s a closer look at why PCl5 breaks the octet rule:
Phosphorus’s Electron Configuration: Phosphorus has the electronic configuration [Ne] 3s² 3p³ . It has five valence electrons.
Bonding with Chlorine: Phosphorus forms five single covalent bonds with five chlorine atoms, sharing one electron with each chlorine. This results in ten electrons around phosphorus (five from phosphorus itself and five from chlorine).
Expanding the Octet: Phosphorus’s empty 3d orbitals can accommodate these additional electrons. The five chlorine atoms donate an electron each, forming five sigma bonds with phosphorus.
This process of exceeding the octet rule is referred to as octet expansion. It’s important to note that octet expansion is generally seen in elements in the third period and beyond, as they have available d-orbitals.
Why does PCl5 have an expanded octet?
In its atomic state, phosphorus (P) has five valence electrons. When it bonds with five chlorine (Cl) atoms to form PCl5, phosphorus shares its five valence electrons with the chlorine atoms. This results in phosphorus having a total of ten electrons in its valence shell.
Since phosphorus has more than eight electrons in its valence shell, we call this an expanded octet.
But why does phosphorus do this?
Phosphorus is in the third period of the periodic table. This means it has access to 3d orbitals in addition to its 2s and 2p orbitals. This allows phosphorus to accommodate more than eight electrons in its valence shell by using its 3d orbitals.
So, to summarize: PCl5 has an expanded octet because phosphorus can utilize its 3d orbitals to hold more than eight electrons in its valence shell, allowing it to form five bonds with chlorine atoms. This is a common phenomenon for elements in the third period and beyond, as they have access to d orbitals that enable them to form more bonds and have more than eight electrons in their valence shell.
Is pci5 an exception to the octet rule?
PCl5, or phosphorus pentachloride, has a central phosphorus atom bonded to five chlorine atoms. This structure gives the phosphorus atom ten electrons in its valence shell, exceeding the usual eight that the octet rule prescribes.
So, why does PCl5 break the rule? The answer lies in phosphorus’s ability to expand its valence shell. Phosphorus is in the third period of the periodic table, meaning it has access to d orbitals in its valence shell. These d orbitals allow phosphorus to accommodate more than eight electrons, forming a hypervalent molecule.
It’s important to note that while the octet rule is a helpful guideline for understanding basic bonding, it isn’t a hard and fast rule. Elements in the third period and beyond can sometimes have more than eight electrons in their valence shell due to the availability of d orbitals, making them exceptions to the rule.
Why PCl5 does not obey the octet rule?
Let’s break this down:
The Octet Rule: Most atoms want to have eight electrons in their outer shell to achieve stability. Think of it like a happy family needing eight members to feel complete.
Phosphorus and Chlorine: Phosphorus has five electrons in its outer shell, and chlorine has seven. To reach a stable octet, phosphorus needs three more electrons, and chlorine needs one.
PCl5: The Exception: In PCl5, phosphorus forms five bonds with chlorine. Each bond shares two electrons, so phosphorus ends up with ten electrons in its outer shell, exceeding the octet rule.
You might be wondering how this is possible. It turns out the octet rule isn’t set in stone. While it applies to many elements, some elements, like phosphorus, can expand their outer shell and accommodate more electrons. This is because they have available d-orbitals that can hold additional electrons.
So, while PCl5 is an exception to the octet rule, it’s a great example of how the rules of chemistry can sometimes be flexible. It also shows us that even when things seem unusual, there’s often a logical explanation behind it.
Does PCl obey the octet rule?
Let’s break this down further:
Phosphorus: Phosphorus starts with five valence electrons. By forming three single bonds with chlorine, it gains three more electrons, bringing its total to eight, satisfying the octet rule.
Chlorine: Each chlorine atom starts with seven valence electrons. By forming a single bond with phosphorus, it gains one more electron, bringing its total to eight, also satisfying the octet rule.
In summary: The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight electrons in their outermost shell. In PCl3, both phosphorus and chlorine achieve this stable configuration through the formation of covalent bonds.
This means that PCl3 is a stable molecule that follows the octet rule, which is a fundamental principle in understanding chemical bonding.
See more here: Is Pcl5 A Super Octet? | Does Pcl5 Obey The Octet Rule
Does PC5 obey the octet rule?
Let’s break down why. In a PC5 molecule, each chlorine atom has a complete octet of eight electrons. This means they’re happy and stable. But, phosphorus is a bit different. It has ten electrons surrounding it, not eight. This is because phosphorus can expand its valence shell to accommodate more than eight electrons.
This ability to expand its valence shell is why phosphorus can form PC5. It’s important to note that phosphorus is not the only element that can do this. Elements in the third row and beyond on the periodic table can expand their valence shells.
So, while the octet rule is a helpful guideline for many molecules, it’s not a hard and fast rule for all of them. In the case of PC5, phosphorus is a great example of an element that can break the rule!
Let’s think about it this way: Imagine phosphorus is like a table that can be expanded. It has room for eight people (electrons) but can fit more. In PC5, phosphorus is like a table that’s been expanded to fit ten people. It’s still a stable table, just a bit bigger than the standard eight-person version.
Why does PCl5 break the octet rule?
Phosphorus, being a member of Group 15, is known to expand its octet. This means that phosphorus can accommodate more than eight electrons in its valence shell, unlike most elements in the second period. This is due to the availability of empty d orbitals in the third period and beyond, allowing for the expansion of the octet.
PCl5 exhibits this phenomenon. In PCl5, the central phosphorus atom forms five bonds with five chlorine atoms, resulting in a total of ten electrons surrounding the phosphorus atom. This exceeds the typical eight electrons required for a stable octet.
To understand this further, let’s break down the electron configuration of phosphorus. Phosphorus has the electron configuration [Ne] 3s² 3p³. It has five valence electrons in its outer shell. In PCl5, phosphorus forms five covalent bonds with five chlorine atoms. This means that each chlorine atom contributes one electron to the bond, and phosphorus contributes its five valence electrons. This results in ten electrons surrounding phosphorus, surpassing the octet rule.
The ability to expand the octet arises from the involvement of phosphorus’s 3d orbitals. These d orbitals, being vacant, can participate in bonding. In PCl5, the phosphorus atom uses its 3s, 3p, and 3d orbitals to form five hybrid orbitals, allowing it to bond with five chlorine atoms. This hybridization allows for the expansion of the octet and the formation of the stable PCl5 molecule.
What is an example of the octet rule?
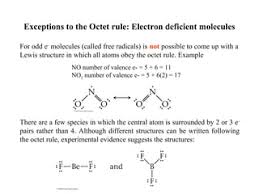
One exception is when there’s an odd number of valence electrons. Let’s take the nitrogen (II) oxide molecule (NO) as an example. Nitrogen has five valence electrons, while oxygen has six. That gives us eleven valence electrons to work with.
It’s impossible to give each atom a full octet with an odd number of electrons. Instead, nitrogen will have seven electrons around it, while oxygen will have eight. This means that nitrogen doesn’t have a full octet, but it’s still a relatively stable molecule. This is a classic example of the octet rule being broken.
Why is this? Well, the octet rule is all about achieving stability. Atoms want to have their outer shells filled with eight electrons because it makes them less reactive. It’s like having a full set of tools in your toolbox – it means you can tackle any job! But when you have an odd number of electrons, it’s like trying to build something with half a nail. You can still get the job done, but it’s not ideal.
There are a couple of reasons why NO can still be stable even though nitrogen doesn’t have a full octet. First, the NO molecule is a radical, meaning it has an unpaired electron. This unpaired electron actually adds a bit of stability to the molecule. Imagine it as a sort of “glue” that holds the molecule together.
Second, the NO molecule has a very strong bond between the nitrogen and oxygen atoms. This strong bond helps to overcome the lack of a full octet on nitrogen. It’s like having a really strong piece of tape that can hold things together even if there are a few gaps.
So, while the octet rule is a great guideline for understanding how atoms bond, it’s important to remember that it’s not always the case. There are some exceptions, like NO, where atoms might not have a full octet, but the molecule is still stable. These exceptions help us to understand the complex world of chemistry and how molecules can form even when the rules are bent a little!
Do odd-electron molecules obey the octet rule?
Let’s dive a little deeper. Three-electron bonds are a fascinating way to understand how molecules with an odd number of electrons can still achieve stability. Imagine a bond where you have three electrons shared between two atoms, instead of the usual two. This arrangement allows each atom to have a filled outer shell, effectively satisfying the octet rule.
Think of it like this: one electron from each atom forms a traditional two-electron bond, leaving one electron to occupy a non-bonding orbital on one of the atoms. This unpaired electron can then be shared with another unpaired electron on a neighboring atom, forming a three-electron bond.
For example, nitrogen dioxide (NO2) is an odd-electron molecule with a total of 17 valence electrons. Its Lewis structure shows one nitrogen atom with only seven valence electrons, making it seem like it doesn’t follow the octet rule. However, if you consider the three-electron bond, the nitrogen atom actually has a total of eight electrons surrounding it, fulfilling the octet rule.
It’s important to remember that the octet rule is a simplification, a helpful guideline for predicting molecular structures. While it’s a great starting point, we must understand that there are exceptions, and ab initio molecular orbital calculations are often necessary to truly understand the bonding behavior of molecules.
See more new information: barkmanoil.com
Does Pcl5 Obey The Octet Rule? The Surprising Answer
Let’s dive into the world of chemistry and explore the fascinating compound PCl5, also known as phosphorus pentachloride. One of the first things you’ll learn about PCl5 is that it’s an exception to the octet rule. Let’s break down why.
The Octet Rule
The octet rule is a fundamental concept in chemistry. It states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. This stable configuration resembles that of a noble gas, making the atom less reactive.
The Structure of PCl5
PCl5 has a central phosphorus atom surrounded by five chlorine atoms. The phosphorus atom has five valence electrons, and each chlorine atom has seven valence electrons. To form PCl5, the phosphorus atom needs to form five bonds.
Now here’s where the magic happens! To achieve this, phosphorus must expand its valence shell to accommodate 10 electrons. This is possible because phosphorus has access to empty d orbitals in its valence shell, which allows it to form more than four bonds.
So, the answer to our question is a resounding NO!PCl5 does not obey the octet rule. It’s a classic example of a compound that expands its octet.
Why Does PCl5 Expand Its Octet?
The answer lies in the size and electronegativity of the phosphorus atom. Phosphorus is a large atom with a relatively low electronegativity. This means that it’s more likely to share its electrons with other atoms, even if it means expanding its octet.
Here’s a simplified analogy: imagine a small, tightly packed box. You can only fit so many things inside. Now imagine a larger box. You can fit more things in the larger box, right? The same applies to the phosphorus atom. It’s larger than the other elements in its period, and it has access to those empty d orbitals, giving it more space to accommodate more electrons.
Exceptions to the Octet Rule
PCl5 isn’t the only compound that breaks the octet rule. There are many other exceptions. Here are a few examples:
SF6 (Sulfur hexafluoride): Sulfur has six valence electrons and forms six bonds with six fluorine atoms.
XeF4 (Xenon tetrafluoride): Xenon has eight valence electrons and forms four bonds with four fluorine atoms.
PCl3 (Phosphorus trichloride): Phosphorus has five valence electrons and forms three bonds with three chlorine atoms.
Remember, the octet rule is a guideline, not a strict rule. While it applies to many compounds, it doesn’t hold true for all.
Understanding the Concept
It’s crucial to understand the underlying principles behind the octet rule and its exceptions. This knowledge will help you grasp the behavior of atoms and molecules. You’ll be able to predict and explain chemical reactions and the formation of different compounds.
FAQs
Q: Why is the octet rule so important?
A: The octet rule helps us understand why atoms bond with each other. It explains why certain compounds are stable and why others are reactive. This knowledge is essential for predicting chemical reactions and understanding the properties of substances.
Q: How do I know if a compound obeys the octet rule?
A: Count the number of valence electrons for each atom in the compound. If the central atom has eight electrons in its outer shell, it follows the octet rule.
Q: What are some examples of compounds that obey the octet rule?
A: Many common compounds obey the octet rule, such as water (H2O), methane (CH4), and ammonia (NH3).
Q: What happens when the octet rule is not obeyed?
A: When the octet rule is not obeyed, the compound might be less stable or more reactive. It could also have unusual properties compared to compounds that follow the octet rule.
Q: Can the octet rule be broken for all elements?
A: No. The octet rule is primarily applicable to elements in the second and third periods. Elements in later periods can have more than eight electrons in their valence shell due to the availability of d orbitals.
Q: How do I explain the expanded octet in PCl5?
A: Phosphorus, being a larger atom with access to empty d orbitals, can expand its valence shell to accommodate more than eight electrons. In PCl5, phosphorus forms five bonds with five chlorine atoms, resulting in 10 electrons around the phosphorus atom.
Q: Is the octet rule always applicable?
A: While it’s a useful guideline, it’s not a universal rule. There are exceptions, and it’s essential to understand the factors that lead to those exceptions.
Q: What are the implications of the octet rule in chemical bonding?
A: The octet rule plays a crucial role in understanding chemical bonding and predicting the stability of compounds. It’s a powerful tool for understanding the properties of substances and the reactions they undergo.
Understanding the octet rule, its exceptions, and the factors that influence it is key to understanding the world of chemistry. As you continue your journey through the fascinating realm of chemical bonding, remember that the octet rule is a valuable tool, but it’s not the only rule to follow. Keep an open mind, and you’ll be amazed at the complex and beautiful world of chemistry.
Violations of the Octet Rule – Chemistry LibreTexts
Three cases can be constructed that do not follow the Octet Rule, and as such, they are known as the exceptions to the Octet Rule. Following the Octet Rule for Lewis Dot Structures leads to the most Chemistry LibreTexts
Does PC5 obey the octet rule? | Socratic
The chlorine atoms obey the octet rule, but the phosphorus atom does not. In a molecule of phosphorus pentachloride, PCl_5, each chlorine atom ends up with an Socratic
Why does PCl5 break the Octet Rule? – CHEMISTRY
Since P is the least electro-negative, it will be the central element. Considering that anything from group 3 to 7 in the p block has an expanded octet, which Laurence Lavelle
8.6: Exceptions to the Octet Rule – Chemistry LibreTexts
However, there are three general exceptions to the octet rule: Molecules, such as NO, with an odd number of electrons; Molecules in which one or more atoms possess more than Chemistry LibreTexts
10.1: Lewis Structures and the Octet Rule – Chemistry
Most structures—especially those containing second row elements—obey the octet rule, in which every atom (except H) is surrounded by eight electrons. Exceptions to the octet Chemistry LibreTexts
Exceptions to the octet rule (video) | Khan Academy
While most atoms obey the duet and octet rules, there are some exceptions. For example, elements such as boron or beryllium often form compounds in which the central atom is surrounded by fewer than eight electrons (e.g., BF₃ or BeH₂). Khan Academy
Octet rule – Wikipedia
Although stable odd-electron molecules and hypervalent molecules are commonly taught as violating the octet rule, ab initio molecular orbital calculations show that they largely Wikipedia
Octet Rule Definition, Examples, and Exceptions
The octet rule is a chemistry rule of thumb that says that atoms combine in a way that gives them eight electrons in their valence shells. This achieves a stable electron configuration similar to that of Science Notes and Projects
Lewis Dot of Phosphorous Pentachloride PCl5 – Kentchemistry.com
Lewis Dot of Phosphorous Pentachloride. PCl 5. Back. 70 More Lewis Dot Structures. P does not follow the octet rule. It will hold more than 8 electrons. Phosphorous having Kentchemistry.com
The Octet Rule: Help, Definition, And Exceptions
The Octet Rule Is Not Obeyed In – (1) Co_2 (2) Bcl_3 (3) Pcl_5 (4) Both (2) And (3)
Exceptions To The Octet Rule – Lewis Dot Diagrams
Exceptions To The Octet Rule
Which Does Not Obey The Octet Rule?
Which Of The Following Compounds Does Not Follow The Octet Rule?
What Elements Obey Octet Rule?
How Does Sodium Obey The Octet Rule?
Link to this article: does pcl5 obey the octet rule.
See more articles in the same category here: https://barkmanoil.com/bio