Are you looking for an answer to the topic “what is z in chemistry“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
The atomic number or nuclear charge number (symbol Z) of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (np) or the number of protons found in the nucleus for every atom of that element.Atomic Definitions
Z is used to signify the atomic number or proton number of an atom. Z = # of protons of an atom. A is used to signify the atomic mass number (also known as atomic mass or atomic weight) of an atom. A = # protons + # neutrons.The atomic number, Z, is the number of protons in the nucleus. • This defines the chemical nature of the atom. • It is equal to the total charge on the nucleus.

What does Z stand for in chemistry?
Atomic Definitions
Z is used to signify the atomic number or proton number of an atom. Z = # of protons of an atom. A is used to signify the atomic mass number (also known as atomic mass or atomic weight) of an atom. A = # protons + # neutrons.
What is Z in chemistry energy?
The atomic number, Z, is the number of protons in the nucleus. • This defines the chemical nature of the atom. • It is equal to the total charge on the nucleus.
cis-trans and E-Z naming scheme for alkenes | Alkenes and Alkynes | Organic chemistry | Khan Academy
Images related to the topiccis-trans and E-Z naming scheme for alkenes | Alkenes and Alkynes | Organic chemistry | Khan Academy

What is Z in chemistry class 11?
The effective nuclear charge (often symbolized asZeff or Z*) is the net positive charge experienced by an electron in a multi-electron atom. The term “effective” is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. Chemistry.
Why is atomic number Z?
Why the atomic number is denoted by Z? The atomic number symbol, Z, stands for “Zahl,” meaning German number. The symbol Z denoted an element’s place in the periodic table before 1915.
What does the letter Z represent?
English. In modern English orthography, the letter ⟨z⟩ usually represents the sound /z/. It represents /ʒ/ in words like seizure. More often, this sound appears as ⟨su⟩ or ⟨si⟩ in words such as measure, decision, etc.
What is Z in quantum chemistry?
Z is called the atomic number. The more Z is high, the more complex to study the atom becomes. The electrons interact both with each other and with protons of nucleus.
What is Z of hydrogen?
| General | |
|---|---|
| Names | hydrogen atom, H-1, protium |
| Protons (Z) | 1 |
| Neutrons (N) | 0 |
| Nuclide data |
See some more details on the topic what is z in chemistry here:
Atomic Mass and Mass Number Chemistry Review – ThoughtCo
Z is used to signify the atomic number or proton number of an atom · Z = # of protons of an atom · A is used to signify the atomic mass number ( …
Question: What Is Z In Chemistry – Seniorcareto
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
Why is atomic number represented by Z? | Socratic
The symbol for atomic number, Z, stands for “Zahl”, which means number in German. Prior to 1915, the symbol Z denoted the position of an …
What does Z mean in Chem? – Best Acting Colleges In New York
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every …
What is Z in Bohr’s equation?
B = Bohr’s energy (2.178×10-18 J) Z = charge of the nucleus (H = +1, Li = +3) n = main quantum number; associated to the level of the electron orbit. small value of n = electron closer to the nucleus lower energy level.
How To Calculate The Effective Nuclear Charge of an Electron
Images related to the topicHow To Calculate The Effective Nuclear Charge of an Electron
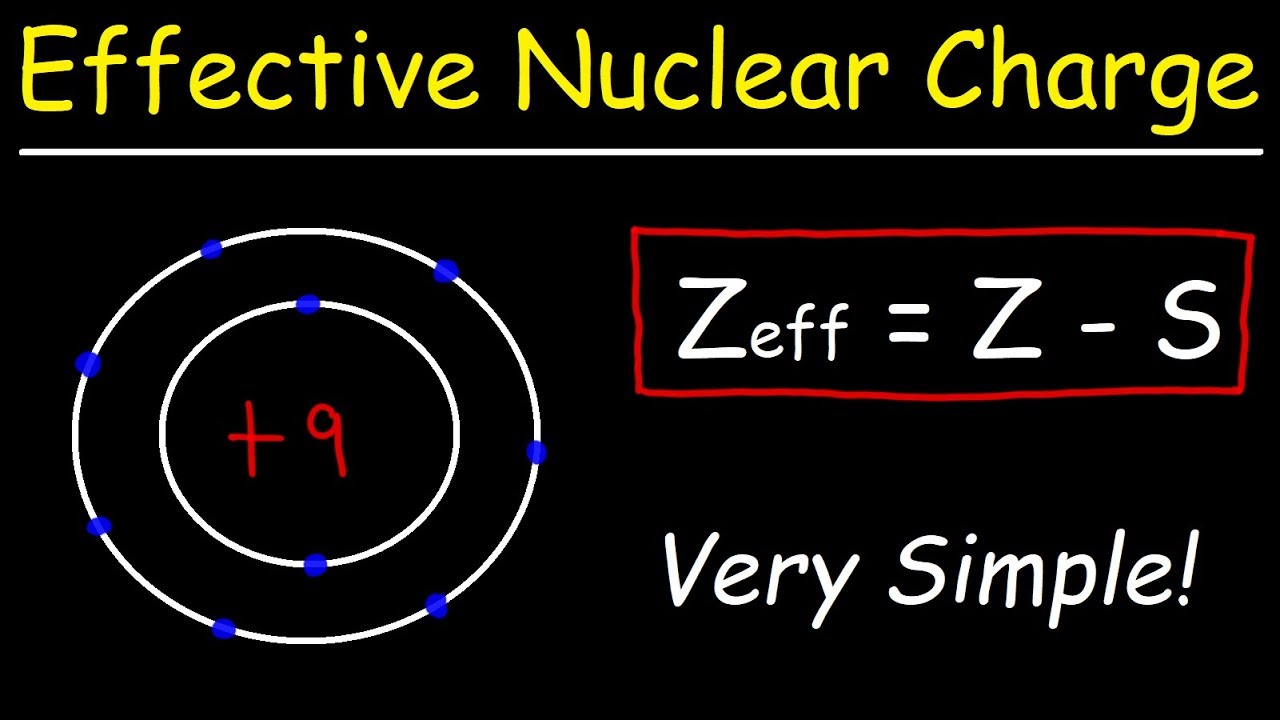
What is Z and A in chemistry?
Z = atomic number = number of protons in the nucleus = number of electrons orbiting the nucleus; A = mass number = number of protons and neutrons in the most common (or most stable) nucleus.
How do you calculate Z effect?
Subtract S from Z
Finally subtract the value of S from Z to find the value of effective nuclear charge, Zeff. For example, Us the Lithium atom, then Z =3 (atomic number) and S = 1.7. Now put the variables in the formula to know the value of Zeff (effective nuclear charge).
What is Z effective of sodium?
Now, for sodium : 1s², 2s², 2p⁶, 3s¹ σ =0.35 ×0 + 0.85 × 8 + 1 × 2 = 0 + 6.8 + 2 = 8.8. So, Zeff = 11 – 8.8 = 2.2.
Is Z the atomic number?
The atomic number (represented by the letter Z) of an element is the number of protons in the nucleus of each atom of that element.
Which number is expressed by Z?
This number is known as the atomic number, which identifies the number of protons in the nucleus of ALL atoms in a given element. The symbol for the atomic number is designated with the letter Z. For example, the atomic number (z) for sodium (Na) is 11. That means that all sodium atoms have 11 protons.
Which group of the periodic table contains element Z?
zinc group element, any of the four chemical elements that constitute Group 12 (IIb) of the periodic table—namely, zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn).
What does Ze mean in physics?
Atoms can be arranged in the Periodic Table. Atomic nuclei have a charge +Ze, where e is the magnitude of the charge on an electron and Z is the atomic number. Z determines the number of atomic electrons and hence the ordered position of that species of atom in the periodic table.
Naming Alkenes Using E Z System – IUPAC Nomenclature
Images related to the topicNaming Alkenes Using E Z System – IUPAC Nomenclature

How do you find the Z component of angular momentum?
The direction of angular momentum is quantized, in that its component along an axis defined by a magnetic field, called the z-axis is given by Lz=mlh2π(ml=−l,−l+1,…,−1,0,1,…l−1,l) L z = m l h 2 π ( m l = − l , − l + 1 , … , − 1 , 0 , 1 , … l − 1 , l ) , where Lz is the z-component of the angular momentum and ml is the …
What is 1s 2s and 2p for orbitals?
At the first energy level, the only orbital available to electrons is the 1s orbital, but at the second level, as well as a 2s orbital, there are also orbitals called 2p orbitals. A p orbital is rather like 2 identical balloons tied together at the nucleus.
Related searches to what is z in chemistry
- atomic mass
- z in chemistry stands for
- what does z stand for in organic chemistry
- what is z effective in chemistry
- what is z in organic chemistry
- what is z^2 in chemistry
- what is m/z in chemistry
- what is the meaning of a in chemistry
- what is z value in chemistry
- what is z number in chemistry
- what is e and z in organic chemistry
- what does a stand for in chemistry
- what is an isotope
- what is z in chemistry solid state
- what is atomic number
- what is an isotope?
- what is z eff in chemistry
- how to find z in chemistry
- what is m/z in organic chemistry
Information related to the topic what is z in chemistry
Here are the search results of the thread what is z in chemistry from Bing. You can read more if you want.
You have just come across an article on the topic what is z in chemistry. If you found this article useful, please share it. Thank you very much.