Are you looking for an answer to the topic “what is cn in chemistry“? We answer all your questions at the website barkmanoil.com in category: Newly updated financial and investment news for you. You will find the answer right below.
A cyanide is a chemical compound that contains the group C≡N. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.In inorganic cyanides, the cyanide group is present as the anion CN−. Soluble salts such as sodium cyanide and potassium cyanide are highly toxic.Cyanides are chemical compounds which are fast acting poisons with a chemical formula CN−. Cyanides consist of one nitrogen atom and carbon atom connected to each other with a triple bond. It is also known as Cyanide anion or nitrile anion Cyanide ions. Certain algae, fungi, and bacteria can produce cyanide.A nitrile is an organic chemical that contains a cyano functional group (subunit), CN–, in which the carbon and nitrogen atoms have a triple bond i.e. C≡N–. The general chemical formula of a nitrile is RCN, where R is the organic group.
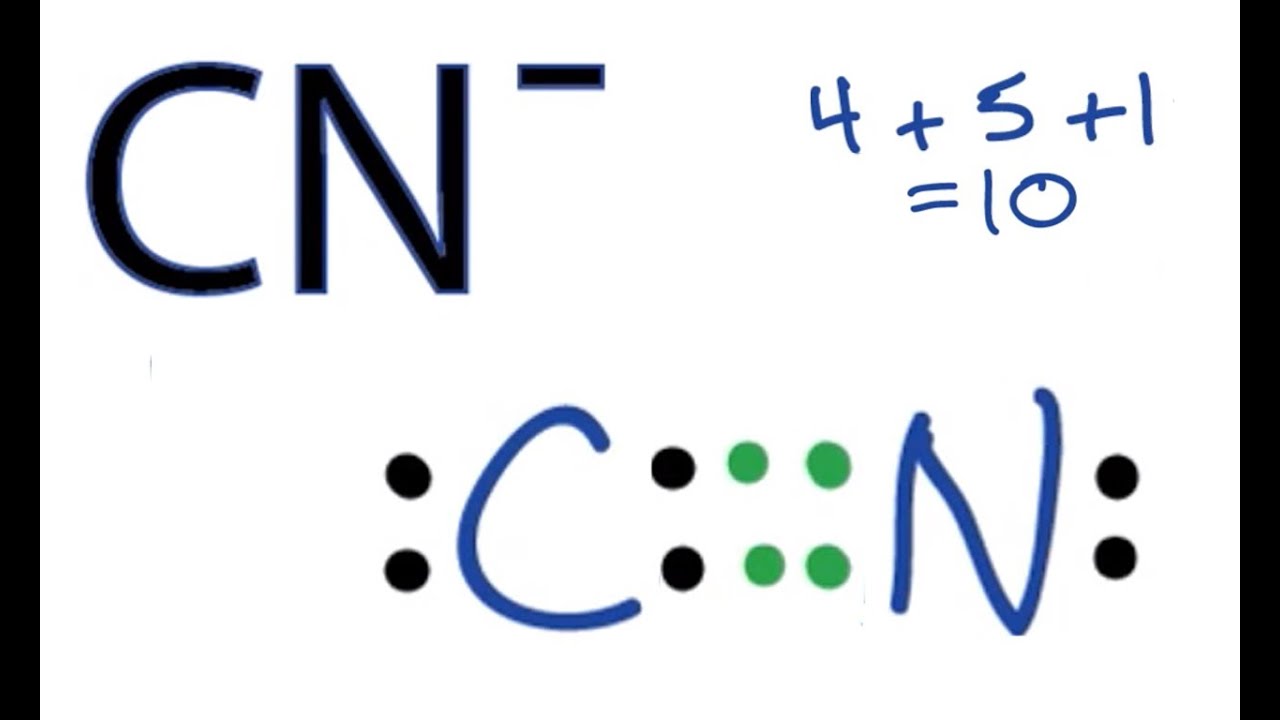
What is a CN ion?
Cyanides are chemical compounds which are fast acting poisons with a chemical formula CN−. Cyanides consist of one nitrogen atom and carbon atom connected to each other with a triple bond. It is also known as Cyanide anion or nitrile anion Cyanide ions. Certain algae, fungi, and bacteria can produce cyanide.
What is CN in organic?
A nitrile is an organic chemical that contains a cyano functional group (subunit), CN–, in which the carbon and nitrogen atoms have a triple bond i.e. C≡N–. The general chemical formula of a nitrile is RCN, where R is the organic group.
CN- Lewis Structure: How to Draw the Dot Structure for the CN-
Images related to the topicCN- Lewis Structure: How to Draw the Dot Structure for the CN-
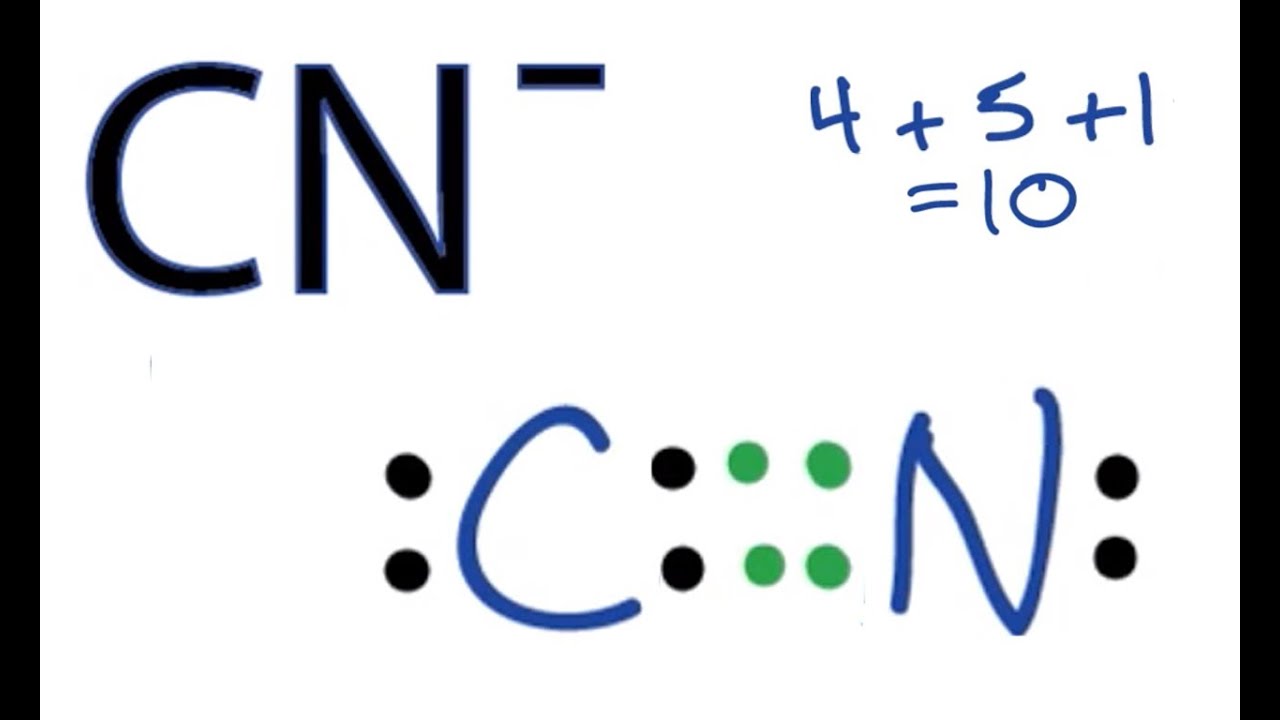
What has a chemical formula of CN+?
Nitridocarbon(1+) | CN+ – PubChem.
What is CN called?
…
Cyanide.
| Names | |
|---|---|
| Chemical formula | CN − |
| Molar mass | 26.018 g·mol−1 |
| Conjugate acid | Hydrogen cyanide |
What is the name for CN −?
A cyanide is a chemical compound that contains the group C≡N. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.In inorganic cyanides, the cyanide group is present as the anion CN−. Soluble salts such as sodium cyanide and potassium cyanide are highly toxic.
What does CN do in a reaction?
This special reaction is a nucleophilic addition, where the nucleophilic CN– attacks the electrophilic carbonyl carbon on the ketone, following a protonation by HCN, thereby the cyanide anion being regenerated.
What is a CN substituent called?
Cycloalkanes are followed by the word -carbonitrile. The substituent name is cyano.
See some more details on the topic what is cn in chemistry here:
cyanide | Definition, Uses, & Effects – Encyclopedia Britannica
cyanide, any compound containing the monovalent combining group CN. In inorganic cyanides, such as sodium cyanide (NaCN), this group is present as the …
What is the chemical name of Cn? – Quora
A cyanide is a chemical compound that contains the group C≡N. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen …
What does CN mean in Chemistry? – Acronym24.com
CN Meaning in Chemistry · CyanideCyanide is an ion with a -1 charge which contains one atom of carbon bound to one atom of nitrogen. · coperniciumCopernicium is …
Is CN a base?
Cyanide is a pseudohalide anion that is the conjugate base of hydrogen cyanide. It has a role as an EC 1.9. 3.1 (cytochrome c oxidase) inhibitor. It is a conjugate base of a hydrogen cyanide and a hydrogen isocyanide.
Worked example: Lewis diagram of the cyanide ion (CN⁻) | AP Chemistry | Khan Academy
Images related to the topicWorked example: Lewis diagram of the cyanide ion (CN⁻) | AP Chemistry | Khan Academy

How do you make CN?
CaO + 2C + N2 = CaCN2 + CO. By heating the product at a high temperature with a further quantity of carbon, with the addition of salt-to prevent frothing and facilitate the reaction, the cyanamide is converted into cyanide as follows: CaCN2 + C = Ca(CN)2.
What is CN charge?
The charge distribution on the cyanide ion has been studied by molecular-orbital calculations. The probable charges on the carbon and nitrogen atoms in [CN]– are –0.40 and –0.60 electron units, respectively.
What is the atomic number of CN?
What element is cyanide?
cyanide Any chemical compound containing a pairing of carbon and nitrogen, but especially sodium cyanide (NaCN).
What is cyanide powder used for?
In manufacturing, cyanide is used to make paper, textiles, and plastics. It is present in the chemicals used to develop photographs. Cyanide salts are used in metallurgy for electroplating, metal cleaning, and removing gold from its ore. Cyanide gas is used to exterminate pests and vermin in ships and buildings.
Is CN a nucleophile?
CN- is an organometallic compound but it can only be used as a nucleophile. nucleophile is a species (an ion or a molecule) which is strongly attracted to a region of positive charge in something else.
Is CN a strong nucleophile?
Cyanide is a fairly strong nucleophile, so the former path should be favored. H will not have this allylic enhancement, so a mixture of substitution (SN2) and elimination (E2) is expected.
CN- Lewis Structure (Cyanide)
Images related to the topicCN- Lewis Structure (Cyanide)

What does NaCN reagent do?
Cyanide adds to aldehydes and ketones to give a cyanohydrin. The reaction is usually carried out using NaCN or KCN with HCl. HCN is a fairly weak acid, but very toxic.
How do you make HCN in situ?
Hydrogen cyanide (HCN) is hazardous to handle because it is highly toxic. Therefore in many syntheses of cyanohydrins, HCN is created in situ by adding a strong acid to a mixture of sodium cyanide and the carbonyl compound, so that hydrogen cyanide is generated in situ.
Related searches to what is cn in chemistry
- cyanure
- Hydrogen cyanide
- Cyanure
- cyanide pubchem
- cn chemical symbol
- Công thức Lewis của cn
- thiocyanate
- hydrogen cyanide
- potassium cyanide
- cong thuc lewis cua cn
- what element is cn
- acetonitrile
- what is nn in chemistry
- Cyanide
- Cyanide pubchem
- Potassium cyanide
- cyanide
- what is cn element
- what is cn in organic chemistry
Information related to the topic what is cn in chemistry
Here are the search results of the thread what is cn in chemistry from Bing. You can read more if you want.
You have just come across an article on the topic what is cn in chemistry. If you found this article useful, please share it. Thank you very much.